Abstract
Actigraphy has been used for more than 60 years to objectively measure sleep–wake rhythms. Improved modern devices are increasingly employed to diagnose sleep medicine disorders in the clinical setting. Although less accurate than polysomnography, the chief advantage of actigraphs lies in the cost-effective collection of objective data over prolonged periods of time under everyday conditions. Since the cost of wrist actigraphy is not currently reimbursed, this method has not enjoyed wide acceptance to date. The present article provides an overview of the main clinical applications of actigraphy, including the recommendations of specialist societies.
Zusammenfassung
Seit mehr als 60 Jahren wird die Aktigraphie eingesetzt, um den Schlaf-Wach-Rhythmus objektiv zu erfassen. Zunehmend werden verbesserte moderne Geräte angewendet, um schlafmedizinische Erkrankungen im klinischen Rahmen zu diagnostizieren. Aktigraphen sind zwar weniger genau als die Polysomnographie, aber ihr größter Vorteil liegt in der kostengünstigen Sammlung objektiver Daten über längere Zeiträume unter Alltagsbedingungen. Da die Kosten der Handgelenksaktigraphie derzeit nicht erstattet werden, hat dieses Verfahren bisher keine breite Akzeptanz erlangt. In der vorliegenden Arbeit wird ein Überblick über die wesentlichen klinischen Anwendungen der Aktigraphie sowie über die Empfehlungen der Fachgesellschaften gegeben.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Behavior in the home setting plays an important role in many sleep-related disorders. Despite the availability of actigraphy, longitudinal disease and treatment courses are usually recorded using subjective reports (e.g., sleep logs/sleep diaries).
Actigraphy has been used for more than 60 years to objectively measure sleep–wake rhythms [1]. The procedure makes it possible to measure and evaluate movement and other parameters such as light exposure over a prolonged period of time.
In recent years, actigraphy has been increasingly used in the clinical setting. Modern medical actigraphs are more accurate and reliable due their improved piezoelectric motion sensors, lithium batteries, and enhanced storage capacities.
Current devices are able to record motor behavior over periods of up to months. Their improved waterproofing and low weight make them suitable for prolonged use under natural conditions, even on moving surfaces (e.g., on ships) [2]. Thus, insight can be gained into the motor phenomena of activity and rest phases as well as of circadian rhythms.
Since the cost of wrist actigraphy is not reimbursed, this method has not enjoyed wide acceptance to date. The present article provides an overview of its main clinical applications (Table 1).
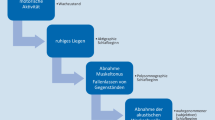
If one puts the main measuring instruments used in sleep medicine in order of accuracy, modern actigraphs rank below the accuracy of polysomnography for the majority of variables measured. Their chief advantage lies in the cost-effective collection of objective data over prolonged periods of time, usually 7–14 days, under everyday conditions. Particularly when investigating insomnia, hypersomnia, and circadian rhythm disorders, longer measurement periods significantly improve validity [4]. For a correct assessment of the sleep onset spectrum, it is important to have a detailed knowledge of the different assessment procedures (Fig. 1). This plays a special role, for example, in the assessment of insomnia patients, as there can be considerable discrepancies between sleep protocols and actigraphy findings.
Sleep onset spectrum. (Adapted from [5]). The recorded sleep duration heavily depends on the measurement method used
Actigraphs
Technical features
Newer actigraphs record motion in up to three axes. The recorded data are usually processed in a frequency range of 0.25–3 Hz with band-pass filters before they are saved. As a general rule, the epochs used in actigraphy devices can be freely selected; in sleep medicine practice, they are usually 30 or 60 s.
For adaptation of the scoring algorithms, empirical values or laboratory standards are used. The fact that there are as yet no standardized scoring recommendations negatively affects the objectivity of scoring and inter-rater reliability [6].
Data acquisition and cleansing
Wrist actigraphy usually measures day–night rhythms in daily life over 1–4 weeks [7]. The ideally waterproof devices are worn on the non-dominant hand for 24 h.
Additional information such as subjective total sleep time and quality can be recorded in a sleep diary. This can be used when discussing findings with the patient, in order to reconstruct everyday situations together with the patient and correlate these with the recorded actigraphy [8].
The use of actigraphy event markers as soon as a sleep attempt begins (eye closure, corresponding to actigraphy light-out) and ends (opening eyes in the morning, getting out of bed, corresponding to actigraphy light-on) has proven successful.
At the same time, sleep opportunity, which is often spent on other activities (e.g., watching TV, reading, eating) can be distinguished from actual sleep attempts. Nighttime sleep interruptions, such as visits to the toilet, are not marked by patients.
Thus, the actigraphy system is able to record the length of sleep, which is needed later to calculate the actigraphy variables (see Table 2; [9]).
After downloading the actigraphy data, manual data cleansing should be performed. This makes it possible to significantly improve the actigraphy report [10]. Sleep-related data are then calculated, and an actogram generated with visualization of the day–night rhythm (see Fig. 2). In clinical practice, automated analysis is generally used to this end [11, 12].
Sample actogram of a care home resident recorded over 1 week. Subjective sleep maintenance disorder and prescription of hypnotics by general practitioner. Actogram interpretation: long main sleep phase with marked nighttime activity, numerous daytime sleep episodes, little light exposure, irregular daytime activity, physiological sleep efficiency. Treatment: restriction of bedtime initiated, age-appropriate daytime activity established, measures taken to activate the patient in the home environment with outdoor activity. WASO wake after sleep onset
The graphic representation of the actogram (see example in Figs. 2 and 3) can be readily used in the consultation process and compared with the patient’s subjective perception.
Extract from 3‑week actigraphy. The social jetlag phenomenon is evident: patient with daytime tiredness and subjectively perceived non-restful sleep. Visibly longer sleep opportunity at weekends. Sleep history with clearly divergent data, sleep diary not reliably kept. The blue triangles represent actigraph event markers placed by the patient at light out and light on. This makes it possible to measure the length of the sleep attempt (sleep opportunity)
Actogram interpretation
An example actogram of a care home resident recorded over a 1‑week period is presented in Fig. 2.
Case study
Actigraphy in social jetlag phenomenon
An extract from a 3-week actigraphy of a patient with social jetlag (SJL) is presented in Fig. 3.
Actigraphy vs. wearables in sleep medicine practice
In sleep medicine consultations, patients often present measurements that have been recorded using smartphones or commercially available wearables.
A number of points need to be considered when interpreting these data:
-
1.
These devices are often unvalidated compared to standard sleep medicine methods (actigraphy or polysomnography) [15].
-
2.
Analysis software is subject to a continuous process of review, hampering its use in research projects [16].
-
3.
The software’s evaluation algorithms are generally not known or cannot be modified [16].
-
4.
At present, professional actigraphy devices are superior to commercially available wearables in terms of data acquisition and security, as well as their evaluation algorithms [17].
-
5.
The sensors used to measure motor activity are not scientifically validated.
-
6.
The threshold values for measuring motor activity differ from manufacturer to manufacturer. Data visualization is not transparent.
-
7.
Statements on sleep structure and quality that are not scientifically validated are often made based on the measurement of movement frequency.
Thus, medical actigraphy devices continue to be recommended for clinical use [18]. Due to the widespread use of wearables and smartphone apps, their validation will remain an important challenge in the coming years for medical societies, some of which have set up their own task forces [19,20,21].
Recommendations
ICSD-3 recommendations on the use of actigraphy
The current version of the International Classification of Sleep Disorders (ICSD-3) recommends actigraphy as a diagnostic tool to supplement classic sleep questionnaires and sleep diaries. Particularly in circadian rhythm disorders does actigraphy have an important role (Table 4).
Recommendations on clinical use of actigraphy (AASM task force)
In 1995 and 2002, the American Academy of Sleep Medicine (AASM) classified actigraphy as a suitable research instrument but deemed its clinical benefit to be unclear. Manual evaluation of actigraphy data was recommended [23, 24].
In 2018, based on current evidence, the AASM issued new recommendations on the use of actigraphy in clinical routine (Table 5; [25]).
Reimbursement aspects
In Germany, actigraphy has not as yet been included in the catalog of services covered by health insurers. To date, it has been billable to the patient as an individual health service (IGeL). A revised catalog of services for privately insured patients includes actigraphy for at least 7 days as a reimbursable service. A policy decision in this regard is pending.
In Switzerland, actigraphy is included in the catalog of mandatory health insurance services, assuming the investigation is carried out at a sleep center recognized by the Swiss Society for Sleep Research, Sleep Medicine, and Chronobiology (SGSSC). If this is not the case, the costs need to be cleared with the health insurance’s medical officer before the investigation is performed.
No provision is made in primary care for the reimbursement of actigraphy in Austria at present.
Discussion
In healthy populations, actigraphy is able to reliably and adequately record total sleep time and sleep onset time within a 24‑h period. Particularly in the case of longitudinal measurements, these results can be placed in a wider context: variances in sleep duration and time of sleep onset; weekly structure (SJL); diagnosis of non-24 syndromes by means of period analysis; estimation of chronotype (relation between time of sleep onset and natural light-dark change [photoperiod] and extent of SJL).
In recent years, actigraphy has been increasingly crystallizing as a clinical tool to diagnose disorders in sleep medicine [26]. In combination with a sleep diary, important information on sleep behavior in the home setting can be obtained over periods of 1–4 weeks with good cost efficiency.
When combined with detailed patient history taking, actigraphy is able to estimate the main sleep-related variables: total sleep time (TST), sleep efficiency (SE), and wake time after sleep onset (WASO) can be determined in numerous sleep disorders.
There are limitations to the use of actigraphy alone for the sleep assessment [27]. It underestimates TST in patients with severe daytime sleepiness, in patients with lower sleep fragmentation, and in patients with more severe sleep-disordered breathing. Actigraphy overestimates TST in patients with high sleep fragmentation, milder severity of sleep-disordered breathing, and short sleepers.
Thus, e.g., motionless wakefulness, as is more common in insomnia patients, is challenging to identify with this method. In order to record the high variability in sleeping patterns in insomnia patients, longer recording periods should be chosen [4].
Actigraphy generally shows good concordance with subjective patient reports on day–night rhythms (e.g., sleep-onset and sleep-offset times). However, there can be significant discrepancy between sleep quality variables such as WASO or SE and the patient’s subjective perception. This mismatch needs to be taken into account at the treatment design stage, in order to be able to develop sustainable treatment plans together with the patients.
The automated evaluation algorithms currently used could be further refined in the future once data collection processes have been adapted to additionally use artificial intelligence [28].
In the authors’ opinion, the factors that remain essential for the successful application of actigraphy in sleep medicine include the clarification of reimbursement issues and the further development of evaluation standards.
References
Tryon WW (1996) Nocturnal activity and sleep assessment. Clin Psychol Rev 16:197–213. https://doi.org/10.1016/0272-7358(95)00059-3
Hein H, Küchler G, Anfang I (2008) Aktographie zur Erkennung von Schlaf-Wach-Rhythmen auf Segelbooten. Somnologie 12:46. https://doi.org/10.1007/s11818-008-1001-0
Ibáñez V, Silva J, Cauli O (2018) A survey on sleep assessment methods. Peer J 6:e4849. https://doi.org/10.7717/peerj.4849
Van Someren, Eus JW (2007) Improving actigraphic sleep estimates in insomnia and dementia: how many nights? J Sleep Res 16:269–275. https://doi.org/10.1111/j.1365-2869.2007.00592.x
Tryon WW (2004) Issues of validity in actigraphic sleep assessment. Sleep 27:158–165
Kawada T (2013) Sleep parameters from actigraphy and sleep diary: is the agreement important for sleep study? Sleep Med 14:298–299. https://doi.org/10.1016/j.sleep.2012.09.016
Zinkhan M, Berger K, Hense S, Nagel M et al (2014) Agreement of different methods for assessing sleep characteristics: a comparison of two actigraphs, wrist and hip placement, and self-report with polysomnography. Sleep Med 15:1107–1114. https://doi.org/10.1016/j.sleep.2014.04.015
Kushida CA, Chang A, Gadkary C, Guilleminault C et al (2001) Comparison of actigraphic, polysomnographic, and subjective assessment of sleep parameters in sleep-disordered patients. Sleep Med 2:389–396
Ancoli-Israel S, Martin JL, Blackwell T, Buenaver L et al (2015) The SBSM guide to actigraphy monitoring: clinical and research applications. Behav Sleep Med 13:S4–S38
Lamp A, Rasmussen I, Cook M, Smith RS et al (2019) 0312 creating A standardized procedure for sleep measured by actigraphy in aviation field studies. Sleep 42:A128–A128. https://doi.org/10.1093/sleep/zsz067.311
Kripke DF, Hahn EK, Grizas AP, Wadiak KH et al (2010) Wrist actigraphic scoring for sleep laboratory patients: algorithm development. J Sleep Res 19:612–619. https://doi.org/10.1111/j.1365-2869.2010.00835.x
Sadeh A (2011) The role and validity of actigraphy in sleep medicine: an update. Sleep Med Rev 15:259–267. https://doi.org/10.1016/j.smrv.2010.10.001
Winnebeck EC, Fischer D, Leise T, Roenneberg T (2018) Dynamics and Ultradian structure of human sleep in real life. Curr Biol 28:49–59.e5. https://doi.org/10.1016/j.cub.2017.11.063
Meltzer LJ (2020) Using actigraphy for the assessment of sleep/wake disorders: C‑06: behavioral sleep medicine: the latest trends and promising developments shaping our practice in the future
Depner CM, Cheng PC, Devine JK, Khosla S et al (2020) Wearable technologies for developing sleep and circadian biomarkers: a summary of workshop discussions. Sleep. https://doi.org/10.1093/sleep/zsz254
Dominick GM, Winfree KN, Pohlig RT, Papas MA (2016) Physical activity assessment between consumer- and research-grade accelerometers: a comparative study in free-living conditions. JMIR Mhealth Uhealth 4:e110. https://doi.org/10.2196/mhealth.6281
Meltzer LJ, Hiruma LS, Avis K, Montgomery-Downs H et al (2015) Comparison of a commercial accelerometer with polysomnography and actigraphy in children and adolescents. Sleep 38:1323–1330. https://doi.org/10.5665/sleep.4918
Insana SP, Gozal D, Montgomery-Downs HE (2010) Invalidity of one actigraphy brand for identifying sleep and wake among infants. Sleep Med 11:191–196. https://doi.org/10.1016/j.sleep.2009.08.010
Scott H, Lack L, Lovato N (2020) A systematic review of the accuracy of sleep wearable devices for estimating sleep onset. Sleep Med Rev 49:101227. https://doi.org/10.1016/j.smrv.2019.101227
de Zambotti M, Cellini N, Menghini L, Sarlo M et al (2020) Sensors capabilities, performance, and use of consumer sleep technology. Sleep Med Clin 15:1–30. https://doi.org/10.1016/j.jsmc.2019.11.003
Bhat S, Ferraris A, Gupta D, Mozafarian M et al (2015) Is there a clinical role for Smartphone sleep Apps? Comparison of sleep cycle detection by a Smartphone application to polysomnography. J Clin Sleep Med 11:709–715. https://doi.org/10.5664/jcsm.4840
American Academy of Sleep Medicine (2014) International classification of sleep disorders Third Edition: ICSD‑3, 3rd edn. American Academy of Sleep Medicine, Darien
Thorpy M, Chesson A, Derderian S (1995) Practice parameters for the use of actigraphy in the clinical assessment of sleep disorders. American Sleep Disorders Association. Sleep 18:285–287. https://doi.org/10.1093/sleep/18.4.285
Littner M, Kushida CA, Anderson WM, Bailey D et al (2003) Practice parameters for the role of actigraphy in the study of sleep and circadian rhythms: an update for 2002. Sleep 26:337–341
Smith MT, McCrae CS, Cheung J, Martin JL et al (2018) Use of actigraphy for the evaluation of sleep disorders and circadian rhythm sleep-wake disorders: an American Academy of Sleep Medicine clinical practice guideline. J Clin Sleep Med 14:1231–1237. https://doi.org/10.5664/jcsm.7230
Stone KL, Ancoli-Israel S (2017) Actigraphy. In: Kryger MH, Roth T, Dement WC (eds) Principles and practice of sleep medicine. Elsevier, Philadelphia, pp 1671–1678.e4 https://doi.org/10.1016/B978-0-323-24288-2.00171-9
Berry RB, Wagner MH (2015) Indications for polysomnography, portable monitoring, and actigraphy. Sleep Med Pearls. https://doi.org/10.1016/B978-1-4557-7051-9.00010-3
Cho T, Sunarya U, Yeo M, Hwang B et al (2019) Deep-ACTInet: end-to-end deep learning architecture for automatic sleep-wake detection using wrist Actigraphy. Electronics 8:1461. https://doi.org/10.3390/ELECTRONICS8121461
Acknowledgement
The publisher would like to thank Christine Rye (Windsor, UK) for translating the article from German into English.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
J. G. Acker, C. Becker-Carus, A. Büttner-Teleaga, W. Cassel, H. Danker-Hopfe, A. Dück, C. Frohn, H. Hein, T. Penzel, A. Rodenbeck, T. Roenneberg, C. Sauter, H.-G. Weeß, J. Zeitlhofer, and K. Richter declare that they have no competing interests.
For this article no studies with human participants or animals were performed by any of the authors. All studies performed were in accordance with the ethical standards indicated in each case.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Acker, J.G., Becker-Carus, C., Büttner-Teleaga, A. et al. The role of actigraphy in sleep medicine. Somnologie 25, 89–98 (2021). https://doi.org/10.1007/s11818-021-00306-8
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11818-021-00306-8
Keywords
- Circadian rhythm
- Sleep-disordered breathing
- Insurance, health
- Polysomnography
- Sleep initiation and maintenance disorders