Abstract
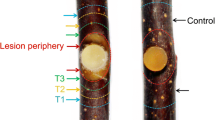
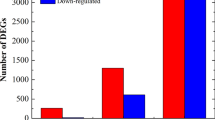
The necrotrophic pathogen Valsa mali (Vm) resulting Valsa canker of apple is considered one of the most destructing fungal diseases. To study the resistance mechanism, we investigated gene expression profiles of suspension cells from resistant varieties ‘Dongbeishanjingzi’ (DS, Malus baccata) and susceptible varieties ‘Gala’ (GL, Malus × domestica) in response to Vm metabolism (VmM) using RNA sequencing (RNA-seq). Functional enrichment showed that differentially expressed genes (DEGs) were widely involved in multiple metabolisms or signals, such as “Lipid metabolic process”, “plant hormone signal transduction” and “plant-pathogen interaction”. Further expressional patterns exhibited that induction of genes related to ‘‘xyloglucan biosynthetic process’’ and ‘‘cell wall biogenesis’’ was beneficial for cell wall integrity and tolerance of DS cells. In brassinosteroid signaling, we identified that TCH4 gene MbTCH4-1 positively regulated Vm resistance of ‘Fuji’ fruit, but BSK gene MdBSK1 negatively regulated the resistance. In contrast, cell death associated with hypersensitive response caused by up-regulation of CNGCs and CDPK genes is an important cause of weakened tolerance in GL cells. Our results provide a new insight direction for the molecular mechanism of apple against Valsa canker.











Similar content being viewed by others
References
Adachi H, Ishihama N, Nakano T, Yoshioka M, Yoshioka H (2016) Nicotiana benthamiana mapk-wrky pathway confers resistance to a necrotrophic pathogen botrytis cinerea. Plant Signal Behav 11(6):00–00
Ajengui A, Bertolini E, Ligorio A, Chebil S, Ippolito A, Sanzani SM (2017) Comparative transcriptome analysis of two citrus germplasms with contrasting susceptibility to phytophthora nicotianae provides new insights into tolerance mechanisms. Plant Cell Rep 37(3):483–499
Bacete L, Mélida H, Mie SE, Molina A (2017) Plant cell wall-mediated immunity: cell wall changes trigger disease resistance responses. Plant J 93(4):614–636
Boudsocq M, Sheen J (2013) CDPKs in immune and stress signaling. Trends Plant Sci 18(1):30–40
Burg V, Uvaprd U, Joosten M, Wit D (2006) Cladosporium fulvum avr4 protects fungal cell walls against hydrolysis by plant chitinases accumulating during infection. Mol Plant Microbe in 19(12):1420–1430
Chen C, Liang W, Li B, Wang C, Dong X (2016) Effects of temperature, humidity, and wound age on valsa mall infection of apple shoot pruning wounds. Plant Dis 100(12):2394–2401
Chen GH, Wang P, Shi L (2021) Review on the dynamic function of cell walls in plant disease resistance. J Inn Mong Agric Univ 42(05):117–120
Clouse SD, Sasse JM (1998) Brassinosteroids: essential regulators of plant growth and development. Annu Rev Plant Biol 49:427–451
Fan WH, Dong XN (2002) In vivo interaction between npr1 and transcription factor tga2 leads to salicylic acid-mediated gene activation in arabidopsis. Plant Cell 14(6):1377–1389
Freymark G, Diehl T, Miklis M, Romeis T, Panstruga R (2007) Antagonistic control of powdery mildew host cell entry by barley calcium-dependent protein kinases (CDPKs). Mol Plant Microbe In 20(10):1213–1221
Fu ZQ, Dong X (2013) Systemic acquired resistance: turning local infection into global defense. Annu Rev Plant Biol 64(1):839–863
Fukushima A, Nakamura M, Suzuki H, Yamazaki M, Knoch E, Mori T, Saito K (2016) Comparative characterization of the leaf tissue of Physalis alkekengi and Physalis peruviana using RNA-seq and metabolite profiling. Front Plant Sci 7:1883
Iliev EA, Wei X, Polisensky DH, Torisky RS, Braam CJ (2002) Transcriptional and posttranscriptional regulation of arabidopsis tch4 expression by diverse stimuli roles of cis regions and brassinosteroids. Plant Physiol 130(2):770–783
Joosten M, Cozijnsen TJ, Wit P (1994) Host resistance to a fungal tomato pathogen lost by a single base-pair change in an avirulence gene. Nature 367(6461):384–386
Kepley JB, Jacobi WR (2000) Pathogenicity of Cytospora fungi on six hardwood species. J Arboric 26(6):326–333
Kohorn BD (2016) Cell wall-associated kinases and pectin perception. J Exp Bot 2:489–494
Livak KJ, Schmittgen TD (2002) Analysis of relative gene expression data using real-time quantitative pcr. Methods 25(4):402–408
Man N, Ding P, Jones J (2022) Thirty years of resistance: zig-zag through the plant immune system. Plant Cell 5(5):7–17
Mao X, Wang C, Lv Q, Tian YZ, Wang DD, Chen BH, Chu MY, Mao J, Li W, Zuo CW (2021) Cyclic nucleotide-gated channel genes (CNGCs) in rosaceae: genome-wide annotation, evolution and the roles on valsa canker resistance. Plant Cell Rep 40(12):2369–2382
Meerloo JV, Kaspers GJ, Cloos J (2011) Cell sensitivity assays the MTT assay. Humana Press, Totowa, pp 237–245
Meng D, Chunlong L, Park HJ, Jonathan G, Wang J, Dandekar AM, Turgeon BG, Cheng L (2018) Sorbitol modulates resistance to alternaria alternata by regulating the expression of an nlr resistance gene in apple. Plant Cell 30(7):1562–1581
Nakashita H, Yasuda M, Nitta T, Asami T (2003) Brassinosteroid functions in a broad range of disease resistance in tobacco and rice. Plant J 33(5):887–898
Ortiz-Morea FA, Liu J, Shan LB, He P (2022) Malectin-like receptor kinases as protector deities in plant immunity. Nat Plants 8(1):27–37
Ping Y, Praz C, Li B, Singla J, Kessel RCB, ScheuermannLüthiOuzunovaErb DLMM (2019) Fungal resistance mediated by maize wall-associated kinase zmwak-rlk1 correlates with reduced benzoxazinoid content. New Phytol 221(2):976–987
Rairdan GJ, Delaney TP (2002) Role of salicylic acid and nim1/npr1 in race-specific resistance in arabidopsis. Genetics 161(2):803–811
Sánchez-Martín J, Keller B (2021) NLR immune receptors and diverse types of non-NLR proteins control race-specific resistance in Triticeae. Curr Opin Plant Biol 62:102053
Stergiopoulos I, de Wit PJ (2009) Fungal effector proteins. Annu Rev Plant Biol 47:233–263
Tarazona S, García F, Ferrer A, Dopazo J, Conesa A (2012) Noiseq: a rna-seq differential expression method robust for sequencing depth biases. Unive Southampt 17(B):18–19
Thaler JS, Humphrey PT, Whiteman NK (2012) Evolution of jasmonate and salicylate signal crosstalk. Trends Plant Sci 17(5):260–270
Vorwerk S, Somerville S, Somerville C (2004) The role of plant cell wall polysaccharide composition in disease resistance. Trends Plant Sci 9(4):203–209
Xu W, Purugganan MM, Polisensky DH, Antosiewicz DM, Fry SC, Braam FJ (1995) Arabidopsis TCH4, regulated by hormones and the environment, encodes a xyloglucan endotransglycosylase. Plant Cell 7(10):1555–1567
Yang CM, Li MQ, Chu CL, Li FQ, Shan CL (2022) Preliminary Study on the Grafting of Sorbus aucuparia by Malus baccata. J Jilin For Sci Technol 51(01):1–3
Ye J (2006) Wego: a web tool for plotting go annotations. Nucleic Acids Res 34:W293–W297
Yin Z, Liu H, Li Z, Ke X, Dou D (2015) Genome sequence of valsa canker pathogens uncovers a potential adaptation of colonization of woody bark. New Phytol 208(4):1202–1216
Zhai L, Zhang M, Lv G, Chen X, Jia N (2014) Biological and molecular characterization of four botryosphaeria species isolated from pear plants showing stem wart and stem canker in china. Plant Dis 98(6):716–726
Zhang Q, Ma C, Zhang Yi GuZ, Li W, Duan X, Li H, Wang Y, Wang S, Li T (2018) A single nucleotide polymorphism in the promoter of a hairpin rna contributes to alternaria leaf spot resistance in apple (malus × domestica borkh). Plant Cell 30(8):1924–1942
Zhao DY, Yuan JC, Xu K, Chen CG, Yan S (2016) Tree morphology, accumulation and distribution characteristics of mineral nutrient in root system of Gala apple young tree with different dwarfing interstocks. Acta Agriculturae Boreali-Sinica 31(04):184–191
Zhao X, Qi C, Jiang H, Zhong M, You C, Li Y, Hao Y (2020) Mdwrky15 improves resistance of apple to botryosphaeria dothidea via the salicylic acid-mediated pathway by directly binding the mdics1 promoter. J Integr Plant Biol 62(4):527–543
Zhou K, Hu L, Li Y, Chen X, Ma F (2019) Mdugt88f1-mediated phloridzin biosynthesis regulates apple development and valsa canker resistance. Plant Physiol 180(4):2290–2305
Zhou H, Bai S, Wang N, Sun X, Dong C (2020) Crispr/cas9-mediated mutagenesis of mdcngc2 in apple callus and vigs-mediated silencing of mdcngc2 in fruits improve resistance to botryosphaeria dothidea. Front Plant Sci 11:1640
Zuo C, Mao J, Chen Z, Chu M, Duo H, Chen B (2018) RNA sequencing analysis provides new insights into dynamic molecular responses to Valsa mali pathogenicity in apple ‘Changfu No. 2’. Tree Genet Genomes 14(5):75.1–75.10
Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (Grant No. 31860545), and Gansu Science and Technology Program (2021QB-030 and 2019C-11).
Author information
Authors and Affiliations
Contributions
CWZ conceived the idea. CWZ, CW, and XM designed the experiments. CW, XM, and DZ performed experiments. CW, XM, HQY, HD, ES, and YL analyzed the data and wrote the manuscript. CW, CWZ, and DZ revised the manuscript. CWZ supervised the study. All authors read the paper and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed consent
The authors declare that they consent to participate to this study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Wang, C., Mao, X., Zhao, D. et al. Transcriptomic analysis reveals that cell wall- and hypersensitive response (HR)-related genes are involved in the responses of apple to Valsa mali. Plant Biotechnol Rep 16, 539–551 (2022). https://doi.org/10.1007/s11816-022-00763-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11816-022-00763-z