Abstract
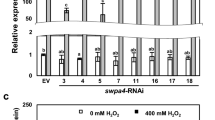
Previously transgenic tobacco plants over-expressing the sweet potato peroxidase gene swpa4 exhibited increased tolerance to various stress conditions. Expression of swpa4 in tobacco also has a positive effect on expression of genes related to reactive oxygen species (ROS) and nitric oxide (NO). Expression levels of respiratory burst oxidase protein D (RBOHD), a major gene regulating ROS, and nitric oxide associated 1 (NOA1), a gene involved in NO generation, are significantly affected by peroxidase inhibitor treatments. In this study, swpa4 was expressed in the rbohD and noa1 knock-out mutants of Arabidopsis, as well as in wild-type plants. The transgenic plants showed increased levels of ROS and NO. Expression of swpa4 activated expression of genes related to ROS and NO. This suggested that swpa4 regulated H2O2 and NO homeostasis, thereby establishing a potential molecular link between the defense signaling pathways mediated by ROS and NO.




Similar content being viewed by others
Abbreviations
- APX:
-
Ascorbate peroxidase
- CAT:
-
Catalase
- NOA:
-
Nitric oxide associated
- RBOH:
-
Respiratory burst oxidase protein
- RNS:
-
Reactive nitrogen species
- ROS:
-
Reactive oxygen species
- POD:
-
Peroxidase
- PR:
-
Pathogenesis-related
- SOD:
-
Superoxide dismutase
References
Alvarez ME, Pennell RI, Meijer P-J, Ishikawa A, Dixon RA, Lamb C (1998) Reactive oxygen intermediates mediate a systemic signal network in the establishment of plant immunity. Cell 92:773–784
Bindschedler LV, Minibayeva F, Gardner SL, Gerrish C, Davies DR, Bolwell GP (2001) Early signaling events in the apoplastic oxidative burst in suspension cultured French bean cells involved cAMP and Ca2+. New Phytol 151:185–194
Bolwell GP, Davies DR, Gerrish C, Auh C-K, Murphy TM (1998) Comparative biochemistry of the oxidative burst produced by rose and French bean cells reveals two distinct mechanisms. Plant Physiol 116:1379–1385
Bolwell GP, Bindschedler LV, Blee KA, Butt VS, Davies DR, Gardner SL, Gerrish C, Minibayeva F (2002) The apoplastic oxidative burst in response to biotic stress in plants: a three component system. J Exp Bot 53:1367–1376
Bowler C, Fluhr R (2000) The role of calcium and activated oxygen as signals for controlling cross-tolerance. Trend Plant Sci 5:241–245
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Choi HW, Kim YJ, Lee SC, Hong JK, Hwang BK (2007) Hydrogen peroxide generation by the pepper extracellular peroxidase CaPO2 activates local and systemic cell death and defence response to bacterial pathogens. Plant Physiol 145:890–904
Clough SJ, Bent AF (1998) Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J 16:735–743
Desikan R, Hancock JT, Coffey MJ, Neill SJ (1996) Generation of active oxygen in elicited cells of Arabidopsis thaliana is mediated by a NADPH oxidase-like enzyme. FEBS Lett 382:213–217
Desikan R, Reynolds A, Hancock JT, Neill SJ (1998) Harpin and hydrogen peroxide both initiate programmed cell death but have differential effects on gene expression in Arabidopsis suspension cultures. Biochem J 330:115–120
Desikan R, Neill SJ, Hancock JT (2000) Hydrogen peroxide induced gene expression in Arabidopsis thaliana. Free Rad Biol Med 28:773–778
Durner J, Klessig DF (1999) Nitric oxide as a signal in plants. Curr Opin Plant Biol 2:369–374
Gas E, Flores-Perez U, Sauret-Gueto S, Rodriguez-Concepcion M (2009) Hunting for plant nitric oxide synthase provides new evidence of a central role for plastids in nitric oxide metabolism. Plant Cell 21:18–23
Kawano T (2003) Role of the reactive oxygen species generating peroxidase reactions in plant defence and growth induction. Plant Cell Rep 21:829–837
Kim YH, Kim CY, Song WK, Park DS, Kwon SY, Lee HS, Bang JW, Kwak SS (2008) Overexpression of sweet potato swpa4 peroxidase results in increased hydrogen peroxide production and enhances stress tolerance in tobacco. Planta 227:867–881
Kim YH, Park SC, Yun BW, Kwak SS (2017) Overexpressing sweetpotato peroxidase gene swpa4 affects nitric oxide production by activating the expression of reactive oxygen species- and nitric oxide-related genes in tobacco. Plant Physiol Biochem 120:52–60
Kwak SS, Kim SK, Lee MS, Jung KH, Park IH, Liu JR (1995) Acidic peroxidase from suspension cultures of sweetpotato. Phytochemistry 39:981–984
Levine A, Tenhaken R, Dixon R, Lamb C (1994) H2O2 from the oxidative burst orchestrates the plant hypersensitive disease resistance response. Cell 79:583–593
Lum HK, Butt YK, Lo SC (2002) Hydrogen peroxide induces a rapid production of nitric oxide in mung bean (Phaseolus aureus). Nitric Oxide 6:205–213
Moreau M, Lee GI, Wang Y, Crane BR, Klessig DF (2008) AtNOS/AtNOA1 is a functional Arabidopsis thaliana cGTPase and not a nitric-oxide synthase. J Biol Chem 283:32957–32967
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant 15:473–497
Neill SJ, Desikan R, Clarke A, Hurst RD, Hancock JT (2002) Hydrogen peroxide and nitric oxide as signalling molecules in plants. J Exp Bot 53:1237–1247
Neill SJ, Desikan R, Hancock JT (2003) Nitric oxide signaling in plants. New Phytol 159:11–35
Parani M, Rudrabhatla S, Myers R, Weirich H, Smith B, Leaman DW, Goldman SL (2004) Microarray analysis of nitric oxide responsive transcripts in Arabidopsis. Plant Biotech J 2:359–366
Polverari A, Molesini B, Pezzotti M, Buonaurio R, Marte M, Delledonne M (2003) Nitric oxide-mediated transcriptional changes in Arabidopsis thaliana. Mol Plant Micobe Int 16:1094–1105
Tossi V, Amenta M, Lamattina L, Cassia R (2011) Nitric oxide enhances plant ultraviolet-B protection up-regulating gene expression of the phenylpropanoid biosynthetic pathway. Plant, Cell Environ 34:909–921
Van Camp W, Van Montagu M, Inze D (1998) H2O2 and NO: redox signals in disease resistance. Trend Plant Sci 3:330–334
Van Ree K, Gehl B, Chehab EW, Tsai YC, Braam J (2011) Nitric oxide accumulation in arabidopsis is independent of NOA1 in the presence of sucrose. Plant J 68:225–233
Vandelle E, Poinssot B, Wendehenne D, Bente´jac M, Pugin A (2006) Integrated signaling network involving calcium, nitric oxide, active oxygen species but not mitogen-activated protein kinases in BcPG1-elicited grapevine defences. Mol Plant Micobe Int 19:429–440
Zemojtel T, Fröhlich A, Palmieri MC, Kolanczyk M, Mikula I, Wyrwicz LS, Wanker EE, Mundlos S, Vingron M, Martasek P, Durner J (2006) Plant nitric oxide synthase: a never-ending story? Trend Plant Sci 11:524–525
Acknowledgements
This research was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT and Future Planning (NRF-2018R1A1A1A05018446), and the KRIBB Initiative Program.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kim, YH., Yun, BW. & Kwak, SS. Expression of the sweet potato peroxidase gene swpa4 in Arabidopsis activates defense genes mediated by reactive oxygen species and nitric oxide. Plant Biotechnol Rep 13, 329–336 (2019). https://doi.org/10.1007/s11816-019-00546-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11816-019-00546-z