Abstract
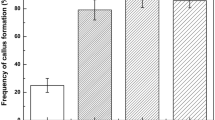
The efficient plant regeneration system from embryogenic cell suspension cultures of Gynura procumbens (Lour.) Merr. is described. Leaf, stem and petiole explants were cultured on Murashige and Skoog (MS) medium supplemented with 2,4-dichlorophenoxyacetic acid (2,4-D) in various concentrations (0, 0.1, 0.3, 1.0 and 3.0 mg l−1). Leaf, stem and petiole explants formed pale-yellow nodular callus and off-white calluses at a frequency of 100% when cultured on MS medium supplemented with more than 1 mg l−1 of 2,4-D after 4 weeks incubation. However, only 20% of pale-yellow nodular callus derived from petiole explants developed into white embryonic structures. Upon transfer to MS basal medium without growth regulators, these white embryonic structures differentiated into somatic embryos. Embryogenic cell suspension cultures were initiated from petiole-derived pale-yellow nodular calluses. More than 73.2% of regenerated plantlets via somatic embryogenesis produced roots on MS medium supplemented with 0.1 mg l−1 α-naphthaleneacetic acid and 1 mg l−1 indole-3-butyric acid (IBA), respectively. Rooted plantlets were successfully transplanted to soil mixture of sterile vermiculite and potting soil (1:1) and grown to maturity in a growth chamber, achieving a survival rate of > 95%. The plant regeneration system from embryogenic cell suspension cultures of G. procumbens established in this study could be applied as an alternative for mass proliferation as well as molecular breeding for quality improvement of G. procumbens.



Similar content being viewed by others
References
Akowuah GA, Sadikun A, Mariam A (2002) Flavonoid identification and hypoglycaemic studies of the butanol fraction from Gynura procumbens. Pharm Biol 40:405–410
Alizah Z, Nurulaishah Y (2015) Multiple shot regeneration from nodal explants of Gynura procumbens (Lour.) Merr. Annu Res Rev Biol 6:85–88
Chan LK, Lim SY, Pan LP (2009) Micropropagation of Gynura procumbens (Lour.) Merr. an important medicinal plant. J Med Plant Res 3:105–111
Dai N, Yu YC, Ren TH, Wu JG, Jiang Y, Shen LG, Zhang J (2007) Gynura root induces hepatic veno-occlusive disease: a case report and review of the literature. World J Gastroenterol 13:1628–1631
De-la-Peña C, Nic-Can GI, Galaz-Ávalos RM, Avilez-Montalvo R, Loyola-Vargas VM (2015) The role of chromatin modifications in somatic embryogenesis in plants. Front Plant Sci 6:635
Hew CS, Khoo BY, Gam LH (2013) The anti-cancer property of proteins extracted from Gynura procumbens (Lour.) Merr. PLoS One 8:7 e68524
Hoe SZ, Kamaruddin MY, Lam SK (2007) Inhibition of angiotensin-converting enzyme activity by a partially purified fraction of Gynura procumbens in spontaneously hypertensive rats. Med Princ Pract 16:203–208
Iskander MN, Song Y, Coupar IM, Jiratchariyakul W (2002) Anti-inflammatory screening of the medicinal plant Gynura procumbens. Plant Food Hum Nutr 57:233–244
Jie EY, Ryu YB, Choi SA, Ahn MS, Liu JR, Min SR, Kim SW (2015) Mass propagation of microtubers from suspension cultures of Pinellia ternata cells and quantitative analysis of succinic acid in Pinellia tubers. Plant Biotechnol Rep 9:331–338
Kaewseejan N, Sutthikhum V, Siriamornpun S (2015) Potential of Gynura procumbens leaves as source of flavonoid-enriched fractions with enhanced antioxidant capacity. J Funct Foods 12:120–128
Keng CL, Yee LS, Pin PL (2009) Micropropagation of Gynura procumbens (Lour.) Merr. an important medicinal plant. Med Plant Res 3:105–111
Ling APK, Chin MF, Hussein S (2009) Adventitious root production of Centella asiatica in response to plant growth regulator and sucrose concentrations. Med Aromat Plant Sci Biotechnol 3:36–41
Min SR, Liu JR, Kim SW (2007) Plant regeneration from zygotic embryo-derived embryogenic cell suspension cultures of Ranunculus kazusensis. Plant Biotechnol Rep 1:57–60
Mithila J, Hall JC, Victor JMR, Saxena PK (2003) Thidiazuron induces shoot organogenesis at low concentrations and somatic embryogenesis at high concentrations on leaf and petiole explants of African violet (Saintpaulia ionantha Wendl). Plant Cell Rep 21:408–414
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant 15:473–497
Oh MJ, Na HR, Choi HK, Liu JR, Kim SW (2008) High frequency plant regeneration from zygotic embryo derived embryogenic cell suspension cultures of watershield (Brasenia schreberi). Plant Biotechnol Rep 2:87–92
Oh MJ, Ahn MS, Jie EY, Liu JR, Min BW, Kim SW (2013) High-frequency plant regeneration from immature zygotic embryo cultures of Houttuynia cordata Thunb via somatic embryogenesis. Plant Biotechnol Rep 7:527–534
Parvin F, Md JI, Jahan N, Khan H, Md PES, Md AI, Rahaman MH, Md MR (2014) Efficient in vitro micropropagation of Gynura procumbens—an important rare medicinal plant, through shoot tip and nodal segments explants. J Res Biol 4:1444–1450
Perry LM, Metzger J (1980) Medicinal plants of East and South East Asia: attributed properties and uses. The MIT Press, Cambridge
Puangpronpitag D, Kaewseejan N, Nakornriab M (2012) Evaluation of phytochemical composition and antibacterial property of Gynura procumbens extract. Asian J Plant Sci 11:77–82
Qi X, Wu B, Cheng Y, Qu H (2009) Simultaneous characterization of pyrrolizidine alkaloids and N-oxides in Gynura segetum by liquid chromatography/ion trap mass spectrometry. Rapid Commun Mass Spectrom 23:291–302
Saiman MZ, Mustafa NR, Schulte AE, Verpoorte R, Choi YH (2012) Induction, characterization, and NMR-based metabolic profiling of adventitious root cultures from leaf explants of Gynura procumbens. Plant Cell Tissue Organ Cult 109:465–475
Rosidah, Yam MF, Sadikun A, Ahmad M, Akowuah GA, Asmawi MZ (2009) Toxicology evaluation of standardized methanol extract of Gynura procumbens. J Ethnopharmacol 123:244–249
Zahra AA, Kadir FA, Mahmood AA, Al hadi AA, Suzy SM, Sabri SZ, Latif II, Ketuly KA (2011) Acute toxicity study and wound healing potential of Gynura procumbens leaf extract in rats. J Med Plant Res 5:2551–2558
Zhao J, Cui J, Liu J, Liao F, Henny RJ, Chen J (2012) Direct somatic embryogenesis from leaf and petiole explants of Spathiphyllum ‘Supreme’ and analysis of regenerants using flow cytometry. Plant Cell Tissue Organ Cult 110(2):239–249
Acknowledgements
This work was supported by a grant from the KRIBB Research Initiative Program (KGM5281711).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jie, E.Y., Atong, N.S., Ahn, W.S. et al. High-frequency plant regeneration from embryogenic cell suspension cultures of Gynura procumbens. Plant Biotechnol Rep 13, 27–33 (2019). https://doi.org/10.1007/s11816-018-0507-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11816-018-0507-6