Abstract
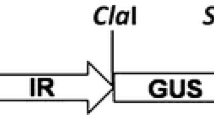
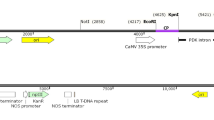
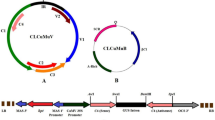
Potato apical leaf curl disease is an emerging geminiviral disease in tropics and subtropics. It was reported for the first time in the year 1999 in northern plains of India but quickly spread to almost all potato growing regions of the country largely due to prevalence of warmer weather during early crop growth, thereby favoring whitefly vector. The problem of apical leaf curl disease in India became more severe due to lack of seed indexing for this virus in conventional seed production scheme. Although it accounts for major yield loss, there is no conventional source of resistance available in potato against Tomato Leaf Curl New Delhi Virus-Potato (ToLCNDV-Potato) that causes this disease in potato. In the present study, we have investigated the potential use of RNAi for obtaining resistance against this DNA virus in potato. The replication-associated protein gene (AC1) of the virus was used to obtain pathogen-derived resistance. The AC1 gene was PCR amplified from field-infected potato leaves, cloned and sequenced (JN393309). It showed 93% sequence similarity with the AC1 gene of Tomato Leaf Curl Virus-New Delhi (TOLCV-NDe; DQ169056) virus. Transgenic plants encoding the AC1 gene in three different orientations, viz. sense, antisense and hairpin loop, were raised. Transgenic lines when challenge inoculated with ToLCNDV-Potato showed different levels of resistance for all three constructs. Transgene integration and copy number in selected transgenic lines were determined by qPCR and further confirmed by Southern blot analysis. Though a reduction in viral titer was observed in transgenic lines encoding either antisense or hairpin loop constructs of AC1 gene, the latter transgenics showed most significant results as shown by reduction in the level of symptom expression in glasshouse screening as well as real-time data of in vivo virus concentration. In fact, we obtained a few totally asymptomatic transgenic lines with hairpin loop strategy.




Similar content being viewed by others
References
Aida R, Kishimoto S, Tanaka Y, Shibata M (2000) Modification of flower colour in torenia (Torenia fournieri Lind.) by genetic transformation. Plant Sci 153:33–42
Altschul FS, Madden LT, Schaffer AS, Zhang J, Zhang Z, Lipman JD (1997) Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acid Res 25:3389–3402
Antignus Y, Vunsh R, Lachman O, Pearlsman M, Maslenin L, Hananya U, Rosner A (2004) Truncated Rep gene originated from tomato yellow leaf curl virus-Israel [Mild] confers strain-specific resistance in transgenic tomato. Ann Appl Biol 144:39–44
Baulcombe DC (1994) Replicase-mediated resistance: a novel type of virus resistance in transgenic plants. Trends Microbiol 2:60–63
Baulcombe D (1996) Mechanisms of pathogen-derived resistance to viruses in transgenic plants. Plant Cell 8:1833–1844
Baulcombe D (2002) RNA silencing. Curr Biol 12:R82–R84
Beachy RN (1993) Introduction: transgenic resistance to plant viruses. Semin Virol 4:327–328
Bradeen JM, Iorizzo M, Mollov DS, Raasch J, Kramer LC, Millett BP et al (2009) Higher copy numbers of the potato RB transgene correspond to enhanced transcript and late blight resistance levels. Mol Plant Microbe Interact 22:437–446
Brummell DA, Balint-Kurti PJ, Harpster MH, Palys JM, Oeller PW, Gutterson N (2003) Inverted repeat of a heterologous 3′-untranslated region for high efficiency, high throughput gene silencing. Plant J 33:793–800
Castillo AG, Collinet D, Deret S, Kashoggi A, Bejarano ER (2003) Dual interaction of plant PCNA with geminivirus replication accessory protein (REn) and viral replication protein (Rep). Virology 312:381–394
Chakrabarti SK, Mandaokar AD, Shukla A, Pattanayak D, Naik PS, Sharma RP, Kunlar PA (2000) Bacillus thuringiensis crylAb gene confers resistance to potato against Helicoverpa armigera (Hubner). Potato Res 43:143–152
Chandel RS, Banyal DK, Singh BP, Malik K, Lakra BS (2010) Integrated management of whitefly, Bemisia tabaci (Gennadius) and potato apical leaf curl virus in India. Potato Res J 53:129–139
Chuang CF, Meyerowitz EM (2000) Specific and heritable genetic interference by double stranded RNA in Arabidopsis thaliana. Proc Natl Acad Sci USA 97:4985–4990
Cogoni C, Macino G (2000) Post-transcriptional gene silencing across kingdoms. Curr Opin Genet Dev 10:638–643
Elbashir SM, Lendeckel W, Tuschl T (2001) RNA interference is mediated by 21- and 22-nucleotide RNAs. Genes Dev 15:188–200
Fire A, Xu S, Montgomery MK, Kostas SA, Driver SE, Mello CC (1998) Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans. Nature 391:806–811
Fukusaki EI, Kawasaki K, Kajiyama S, An CI, Suzuki K, Tanaka Y, Kobayashi A (2004) Flower color modulations of Torenia hybrida by downregulation of chalcone synthase genes with RNA interference. J Biotechnol 111:229–240
Garg ID, Khurana SMP, Kumar S (2001) Association of geminivirus with potatoapical leaf curl in India and its a immune electron microscopic detection. J Indian Potato Assoc 28:227–232
Goodwin J, Chapman K, Swaney S, Parks TD, Wernsman EA, Dougherty WG (1996) Genetic and biochemical dissection of transgenic RNA-mediated virus resistance. Plant Cell 8:95–105
Hamilton AJ, Brown S, Yuanhai H, Ishizuka M, Lowe A, Soles AGA, Grierson D (1998) A transgene with repeat DNA causes high frequency, post transcriptional suppression of Acc-oxidase gene expression in tomato. Plant J 15:737–746
Höfgen R, Willmitzer AL (1988) Storage of competent cells for Agrobacterium transformation. Nucleic Acids Res 16:9877
Hong Y, Stanley J (1995) Regulation of African cassava mosaic virus complementary-sense gene expression by N-terminal sequences of the replication-associated protein AC1. J Gen Virol 76:2415–2422
Ifuku K, Yamamoto Y, Sato F (2003) Specific RNA interference in psbP genes encoded by a multigene family in Nicotiana tabacum with a short 3′-untranslated sequence. Biosci Biotechnol Biochem 67:107–113
Ingelbrect IL, Irvine JE, Mirkov ET (1999) Posttranscriptional gene silencing in transgenic sugarcane. Dissection of homology-dependent virus resistance in a monocot that has a complex polyploid genome. Plant Physiol 119:1187–1198
Levin JZ, Framond AJ, Tuttle A, Bauer MW, Heifetz PB (2000) Methods of double stranded RNA-mediated gene inactivation in Arabidopsis and their use to define an essential gene in methionine biosynthesis. Plant Mol Biol 44:759–775
Lindbo JA, Silva-Rosales L, Proebsting WM, Dougherty WG (1993) Induction of a highly specific antiviral state in transgenic plants: implications for regulation of gene expression and virus resistance. Plant Cell 5:1749–1759
Lomonossof GP (1995) Pathogen derived resistance to plant viruses. Annu Rev Phytopathol 33:323–343
Martínez de Alba AE, Flores R, Hernández C (2002) Two chloroplastic viroids induce the accumulation of the small RNAs associated with post-transcriptional gene silencing. J Virol 76:3094–13096
Naik PS, Chakrabarti SK, Sarkar D, Mandaokar A, Chandla VK, Anandkumar P, Sharma RP (1997) An efficient protocol for Agrobacterium tumefaciens mediated transformation and introduction of synthetic Cry1Ab gene into potato. In: National seminar on potato production on strains in low productivity area. OUAT, Bhubaneshwar. Indian Potato Association, Shimla
Nicot N, Hausman FJ, Hoffmann L, Evers D (2005) Housekeeping gene selection for real-time RT-PCR normalization in potato during biotic and abiotic stress. J Exp Bot 56:2907–2914
Sambrook J, Fritschi EF, Maniatis T (1989) Molecular cloning: a laboratory manual. Cold Spring Harbor Laboratory Press, New York
Sharma R, Bhardwaj V, Dalamu D, Kaushik SK, Sharma S, Umamaheshwari R, Baswaraj R, Kumar V, Gebhardt C (2014) Identification of elite potato genotypes possessing multiple disease resistance genes through molecular approaches. Sci Hortic 179:204–211
Sijen T, Wellink J, Hiriart JB, van Kammen A (1996) RNA-mediated virus resistance: role of repeated transgenes and delineation of targeted regions. Plant Cell 8:2277–2294
Simon-Mateo C, Garcia JA (2011) Antiviral strategies in plants based on RNA silencing. Biochim Biophys Acta 1809:722–731
Smith N, Singh S, Wang MB, Stoutjesdijk P, Green A, Waterhouse PM (2000) Total silencing by intron-spliced hairpin RNAs. Nature 407:319–320
Stam M, Mol JNM, Kooter JM (1997) The silence of genes in transgenic plants. Ann Bot 79:3–12
Stam M, de Bruin R, Van Blokland R, van der Hoorn RA, Mol JN, Kooter JM (2000) Distinct features of posttranscriptional gene silencing by antisense transgenes in single copy and inverted T-DNA repeat loci. Plant J 21:27–42
Waterhouse PM, Graham MW, Wang MB (1998) Virus resistance and gene silencing in plants can be induced by simultaneous expression of sense and antisense RNA. Proc Natl Acad Sci USA 96:13959–13964
Wesley V, Helliwell CA, Smith NA, Wang MB, Rouse DT, Liu Q, Gooding PS, Singh SP, Abbott D, Stoutjesdijk PA, Robinson SP, Gleave AP, Green AG, Waterhouse PM (2001) Construct design for efficient, effective and high throughput gene silencing in plants. Plant J 27:581–591
Wilson TMA (1993) Strategies to protect crop plants against viruses, pathogen derived resistance blossoms. Pro Natl Acad Sci USA 90:3134–3141
Witte CP, Tiller S, Isidore E, Davies HV, Taylor M (2005) Analysis of two alleles of the urease gene from potato: polymorphisms, expression, and extensive alternative splicing of the corresponding mRNA. J Expl Bot 56:91–99
Acknowledgements
The authors acknowledge the financial grant received from Indian Council of Agricultural Research, New Delhi, India. We are also thankful to Drs. S. K. Pandey and B. P. Singh, Former Directors, ICAR-CPRI, Shimla, for providing laboratory facilities for conducting this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Garima Tomar, S. K. Chakrabarti, Nitya Nand Sharma, A. Jeevalatha, S. Sundaresha, Kanika Vyas, and Wamik Azmi declare that they have no conflict of interest.
Ethical standards
This article does not contain any studies with human participants or animals performed by any of the authors.
Rights and permissions
About this article
Cite this article
Tomar, G., Chakrabarti, S.K., Sharma, N.N. et al. RNAi-based transgene conferred extreme resistance to the geminivirus causing apical leaf curl disease in potato. Plant Biotechnol Rep 12, 195–205 (2018). https://doi.org/10.1007/s11816-018-0485-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11816-018-0485-8