Abstract
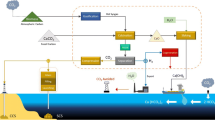
Although various CO2 capture and utilization (CCU) technologies are being researched and developed intensively for the purpose of lowering greenhouse gas emissions, most current technologies remain at low technology readiness levels for industrial use and are less economical compared to conventional processes. Mineral carbonation is a CO2 utilization technology with low net CO2 emissions and high CO2 reduction potential, and various commercialization studies are underway around the world. This manuscript reviews the potential of mineral carbonation as a general CCU technology and the techno-economic and environmental feasibility of a representative technology, which produces sodium bicarbonate through the saline water electrolysis and carbonation steps, and examines the potential CO2 reduction derived from the application of this technology. The future implementation of mineral carbonation technology in ocean alkalinity enhancement for sequestrating atmospheric CO2 or the production of abandoned mine backfill materials is also discussed in order to deploy the technology at much larger scales for a meaningful contribution to the reduction of greenhouse gas emissions.
Similar content being viewed by others
References
M. Mazzotti, J. C. Abanades, R. Allam, K. S. Lackner, F. Meunier, E. Rubin, J. C. Sanchez, K Yogo and R. Zevenhoven, Mineral carbonation and industrial uses of carbon dioxide, in IPCC special report on carbon diox-ide capture and storage, 319 (2005).
C. Song, Catal. Today, 115, 10 (2006).
P. Brinckerhoff, Accelerating the uptake of CCS: Industrial use of captured carbon dioxide, Global CCS Institute (2011).
W. Day, Capture technologies: mineralisation. International symposium on the Sustainable Use of Low Rank Coals, Melbourne (2010).
Long Term Residue Management Strategy, ALCOA (2012).
S. B. AI Yablonsky and David Legere, Decision Point 1 Topical Report, Skyonic Corporation (2013).
C.-H. Huang and C.-S. Tan, Aerosol Air Quality Res., 14, 2 (2014).
S.-Y. Pan, A. Chiang, E-E. Chang, Y.-P. Lin, H. Kim and P.-C. Chiang, Aerosol Air Quality Res., 15, 1072 (2015).
J. H. Lee, J. H. Lee, I. K. Park and C. H. Lee, J. CO2Utilization, 26, 522 (2018).
Putting CO2to use: Creating value from emission, IEA (2019).
J. H. Lee, D. W Lee, C.-Y. Kwak, K.-J. Kang and J. H. Lee, Ind. Eng. Chem. Res., 58, 34 (2019).
CO2/Sodium bicarbonate project, Available from: https://www.twence.com.
Large volume solid inorganic chemicals family: Process BREF for soda ash, European Soda Ash Producers Association (2004).
G. Steinhauser, J. Cleaner Production, 16, 7 (2008).
J. H. Lee, I. K. Park, D. Duchesne, L. Chen, C. H. Lee and J. H. Lee, J. CO2Utilization, 41, 1 (2020).
S. Lindskog and Joseph E. Coleman, Proceedings of the National Academy of Sciences, 70, 9 (1973).
S. Zhang, Z. Zhang, Y. Lu, M. Rostam-Abadi and A. Jones, Bioresour. Technol., 102, 10194 (2011).
A. C. Pierre, Int. Sch. Res. Notices, 2012, 1 (2012).
Y. E. Hwang, K. Kim, H. Seo and D. Y. Koh, ACS Sustain. Chem. Eng., 8, 41 (2020).
J. H. Lee, Techno-economic and environmental evaluation of CO2 mineralization technology based on bench scale experiment, Ph.D. Dissertation, KAIST (2021).
M. M. Al Saadi, E. M. Al Harrsi, Y. G. Mushafri, A. Y. Al Farsi and G. J. Al Maharsi, Global J. Eng. Sci., 1, 3 (2019).
J. Chlistunoff, Final technical report-advanced chlor-alkali technology, Los Alamos National Laboratory (2004).
I. K. Park, C.-Y. Ahn, J. H. Lee, D. W. Lee, C. H. Lee, Y.-H. Cho and Y.-E. Sung, Int. J. Hydrogen Energy, 44, 31 (2019).
L. Lipp, S. Gottesfeld and J. Chlistunoff, J. Appl. Electrochem., 35, 10 (2005).
I. Moussallem, J. Jörissen, U. Kunz, S. Pinnow and T. Turek, J. Appl. Electrochem., 38, 9 (2008).
S. Eggleston, L. Buendia, K. Miwa, T. Ngara and K. Tanabe, IPCC guidelines for national greenhouse gas inventories (2019).
Y.-K. Cho, S.-Y. Nam, Y.-M. Lee, C.-S. Kim, S.-S. Seo, S.-H. Jo, H.-W. Lee and J.-W. Ahn, J. Environm. Sci. Int., 26, 11 (2017).
J.-H. Seo, C.-S. Baek, Y.-J. Kim, M.-K. Choi, K.-H. Cho and J.-W. Ahn, J. Energy Eng., 26, 1 (2017).
J. G. Jang, S. Ji and J.-W. Ahn, J. Korean Inst. Resources Recycling, 26, 2 (2017).
J.G. Jang, J.H. Dae, G. H. Lee, H. J. Lee, J. S. Kim and S. H. Jeong, Economic Evaluation and Commercialization Plan of CO2 Mineralization Technology, Science and Technology Policy Institute (2019).
P. Renforth and G. Henderson, Rev. Geophys., 55, 3 (2017).
H. S. Kheshgi, Energy, 20, 9 (1995).
G. H. Rau, Environm. Sci. Technol., 45, 3 (2011).
J. Lee, M. Park, J. Joo and J.-W. Gil, J. Korean Soc. Environm. Engineers, 39, 3 (2017).
E. S. Rubin, J. E. Davison and H. J. Herzog, Int. J. Greenhouse Gas Control, 40, 378 (2015).
J. H. Lee, N. S. Kwak, H. Niu, J. Wang, S. Wang, H. Shang and S. Gao, Korean Chem. Eng. Res., 58, 1 (2020).
Acknowledgement
This works was supported by the Carbon-to-X (C2X) R&D project (project no. 2020M3H7A1096361) sponsored by the National Research Foundation (NRF) of the Ministry of Science and ICT.
Author information
Authors and Affiliations
Corresponding author
Additional information
Jay H. Lee is currently a KEPCO Chair Professor at Korea Advanced Institute of Science and Technology (KAIST). He is also the director of Saudi Aramco-KAIST CO2 Management Center. He received the AIChE CAST Computing in Chemical Engineering Award and was elected as an IEEE Fellow, an IFAC Fellow, and an AIChE Fellow. He was the 29th Roger Sargent Lecturer in 2016. He published over 200 manuscripts in SCI journals with more than 17,000 Google Scholars citations. His research interests are in the areas of state estimation, model predictive control, planning/scheduling, and reinforcement learning with applications to energy systems and carbon management systems.
Rights and permissions
About this article
Cite this article
Lee, J.H., Lee, J.H. Techno-economic and environmental feasibility of mineral carbonation technology for carbon neutrality: A Perspective. Korean J. Chem. Eng. 38, 1757–1767 (2021). https://doi.org/10.1007/s11814-021-0840-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11814-021-0840-2