Abstract
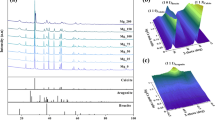
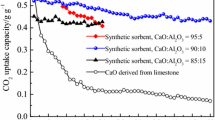
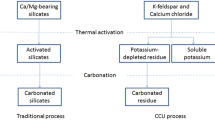
The single process CO2 capture-mineralization approach integrates methods of CO2 absorption using aqueous solvents and mineral carbonation technology to not only remove carbon dioxide quickly, but also to simultaneously produce precipitated calcium carbonate (PCC). To develop a more sustainable process, it is important to extract calcium from inexpensive raw materials such as industrial by-products. The extractant has a significant effect on the quality of the calcium carbonate produced because it determines the anion paired with the calcium cation. In this work, several calcium sources with different anions (Propionate, Acetate, Nitrate and Chloride) were applied in the single process CO2 capture-mineralization method, and their influence on the polymorph of the obtained CaCO3 was investigated. The CaCO3 produced with inorganic calcium sources predominantly exhibited a calcite structure, while the CaCO3 produced with organic calcium sources had a structure in which vaterite and calcite coexist. This result was in good agreement with our DFT calculations, which indicated the adsorption energy of the organic anions (Propionate and Acetate) were lower than the inorganic anions on the surface of vaterite. Except for chloride with its non-polar nature, in most cases, there was a strong correlation between the polymorph and the adsorption energy calculated for each surface. A mechanism for the polymorph CaCO3 formation in our single process CO2 capture-mineralization method was proposed after observing crystal formation at low concentration.
Similar content being viewed by others
References
S. M. Jarvis and S. Samsatli, Renew. Sustain. Energy Rev., 85, 46 (2018).
R. M. Cuéllar-Franca and A. Azapagic, J. CO2 Util., 9, 82 (2015).
A. Sanna, M. Uibu, G. Caramanna, R. Kuusik and M. M. Maroto-Valer, Chem. Soc. Rev., 43, 8049 (2014).
G. A. Bhaduri and L. Šiller, Catal. Sci. Technol., 3, 1234 (2013).
R. Zevenhoven, S. Teir and S. Eloneva, Proc. 19th Int. Conf. Efficiency Costs, Optimization, Simulation and Environmental Impact of Energy Systems, 33, 1661 (2006).
S. M. Lee, S. H. Lee, S. K. Jeong M. H. Youn, D. D. Nguyen, S. W. Chang and S. S. Kim, J. Ind. Eng. Chem., 53, 233 (2017).
Y. Sun, M. S. Yao, J. P. Zhang and G. Yang, Chem. Eng. J., 173, 437 (2011).
Q. Zhao, C. J. Liu, M. F. Jiang, H. Saxén and R. Zevenhoven, Miner. Eng., 79, 116 (2015).
S. Teir, H. Revitzer, S. Eloneva, C. J. Fogelholm and R. Zevenhoven, Int. J. Miner. Process., 83, 36 (2007).
A. H. A. Park and L. S. Fan, Chem. Eng. Sci., 59, 5241 (2004).
M. Ibrahim, M. El-Naas, A. Benamor, S. Al-Sobhi and Z. Zhang, Processes, 7, 115 (2019).
A. Alamdari, A. Alamdari and D. Mowla, J. Ind. Eng. Chem., 20, 3480 (2014).
A. Iizuka, M. Fujii, A. Yamasaki and Y. Yanagisawa, Ind. Eng. Chem. Res., 43, 7880 (2004).
A. M. López-Periago, R. Pacciani, C. García-González, L. F. Vega and C. Domingo, J. Supercrit. Fluids, 52, 298 (2010).
M. Vinoba, M. Bhagiyalakshmi, S. Y. Choi, K. T. Park, H. J. Kim and S. K. Jeong, J. Phys. Chem. C, 118, 17556 (2014).
M. Arti, M. H. Youn, K. T. Park, H. J. Kim, Y. E. Kim and S. K. Jeong, Energy Fuels, 31, 763 (2017).
A. Murnandari, J. M. Kang, M. H. Youn, K. T. Park, H. J. Kim, S. P. Kang and S. K. Jeong, Korean J. Chem. Eng., 34, 935 (2017).
M. Vinoba, M. Bhagiyalakshmi, A. N. Grace, D. H. Chu, S. C. Nam, Y. Yoon, S. H. Yoon and S. K. Jeong, Langmuir, 29, 15655 (2013).
J. M. Kang, A. Murnandari, M. H. Youn, W. Lee, K. T. Park, Y. E. Kim, H. J. Kim, S. P. Kang, J. H. Lee and S. K. Jeong, Chem. Eng. J., 335, 338 (2018).
W. Bao, H. Li and Z. Yi, Ind. Eng. Chem. Res., 49, 2055 (2010).
G. Kresse and D. Joubert, Phys. Rev. B, 59, 1758 (1999).
G. Kresse and J. Furthmüller, Phys. Rev. B, 54, 11169 (1996).
J. P. Perdew, K. Burke and M. Ernzerhof, Phys. Rev. Lett., 78, 1396 (1997).
Y. Liang, A. S. Lea, D. R. Baer and M. H. Engelhard, Surf. Sci., 351, 172 (1996).
A. M. Bano, P. M. Rodger and D. Quigley, Langmuir, 30, 7513 (2014).
S. Grimme, J. Antony, S. Ehrlich and H. Krieg, J. Chem. Phys., 132, 154104 (2010).
H. M. Stowe, L. Vilčiauskas, E. Paek and G. S. Hwang, Phys. Chem. Chem. Phys., 17, 29184 (2015).
J. P. Andreassen, J. Cryst. Growth, 274, 256 (2005).
J. Balmain, B. Hannoyer and E. Lopez, J. Biomed. Mater. Res., 48, 342 (1999).
E. N. Maslen, V. A. Streltsov and N. R. Streltsova, Acta Crystallogr. Sect. B, 49, 636 (1993).
D. M. Duffy and J. H. Harding, J. Mater. Chem., 12, 3419 (2002).
N. H. de Leeuw and S. C. Parker, J Phys. Chem. B, 102, 2914 (1998).
J. S. Lardge, D. M. Duffy and M. J. Gillan, J. Phys. Chem. C, 113, 7207 (2009).
E. Ataman, M. P. Andersson, M. Ceccato, N. Bovet and S. L. S. Stipp, J. Phys. Chem. C, 120, 16586 (2016).
D. V. Okhrimenko, J. Nissenbaum, M. P. Andersson, M. H. M. Olsson and S. L. S. Stipp, Langmuir, 29, 11062 (2013).
D. Kralj, L. Brečević and A. E. Nielsen, J. Cryst. Growth, 104, 793 (1990).
D. Kralj, L. Brecević and A. E. Nielsen, J. Cryst. Growth, 143, 269 (1994).
D. B. Trushina, T. V. Bukreeva, M. V. Kovalchuk and M. N. Antipina, Mater. Sci. Eng. C, 45, 644 (2015).
Acknowledgement
This work was supported by the Energy Demand Side Management Program of the Korea Institute of Energy Technology Evaluation and Planning (KETEP) granted financial resource from the Ministry of Trade, Industry & Energy, Republic of Korea (No. 20182010202100).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Supporting Information
Additional information as noted in the text. This information is available via the Internet at http://www.springer.com/chemistry/journal/11814.
Rights and permissions
About this article
Cite this article
Moon, D.H., Murnandari, A., Salawu, O. et al. Formation of CaCO3 from calcium sources with different anions in single process of CO2 capture-mineralization. Korean J. Chem. Eng. 37, 1709–1716 (2020). https://doi.org/10.1007/s11814-020-0583-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11814-020-0583-5