Abstract
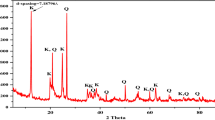
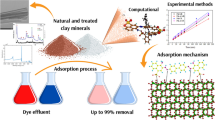
Natural zeolite as a low cost clay was tested for removal of crystal violet known as a noxious dye. Five characterization techniques were used for this study Optimizing and modeling of adsorption were performed at minimum time by an applicable technique named as response surface methodology (RSM). Three effective variables (pH, temperature (T) and adsorbate-to-adsorbent ratio (a/A)) were monitored to obtain the dye removal efficiencies. The maximum removal of dye was obtained at pH=10, T=25°C and a/A=0.1 g/g. For natural zeolite, the Fractal-Langmuir model was selected as an appropriate model for kinetic studies and the Freundlich isotherm was the best isotherm for equilibrium studies. Thermodynamic investigations showed that the adsorption of dye on natural zeolite is endothermic process and a spontaneous reaction. The maximum dye adsorption capacity of natural zeolite and Merck activated carbon on the surface of each adsorbent was obtained at 177.75 and 84.11 mg/g, respectively. In comparison with the maximum adsorption capacity of activated carbon obtained from Merck Company, we can conclude that natural zeolite possesses a higher adsorption capacity. These results reveal that, natural zeolite is an excellent and affordable adsorbent for removal of crystal violet from wastewater as compared to activated carbon.
Similar content being viewed by others
References
O. J. Hao, H. Kim and P. C. Chiang, Environ. Sci. Technol., 30, 449 (2000).
S. Li, Bioresour. Technol., 101, 2197 (2010).
S. Senthilkumaar, P. Kalaamani and C. V. Subburaam, J. Hazard. Mater., 136, 800 (2006).
H. He, S. Yang, K. Yu, Y. Ju and C. S. L. Wang, J. Hazard. Mater., 173, 393 (2010).
L. Guz, G. Curutchet, R. M. Torres Sánchez and R. Candal, J. Environ. Chem. Eng., 2, 2344 (2014).
H. Bashiri and M. Rafiee, J. Saudi Chem. Soc., 20, 474 (2016).
R. Zhu, Q. Chen, H. Liu, F. Ge, L. Zhu, J. Zhu and H. He, Appl. Clay Sci., 88, 33 (2014).
S. Eris and H. Bashiri, Prog. React. Kinet. Mechanism, 41, 109 (2016).
S. V. K. Gupta, Environ. Manage., 90, 2313 (2009).
K. Banerjee, P. N. Cheremisinoff and S. L. Cheng, Water Res., 31, 249 (1997).
K. Y. Hor, J. M. C. Chee, M. N. Chong, B. Jin, C. Saint, P. E. Poh and R. Aryal, J. Cleaner Prod., 118, 197 (2016).
T. Sismanoglu, Y. Kismir and S. Karakus, J. Hazard. Mater., 184, 164 (2010).
I. Humelnicu, A. Băiceanu, M.-E. Ignat and V. Dulman, Process Saf. Environ. Prot., 105, 274 (2017).
M. Qiu, C. Qian, J. Xu, J. Wu and G. Wang, Desalination, 243, 286 (2009).
R. P. Townsend, Chapter 10 Ion Exchange in Zeolites, in: H. van Bekkum, E. M. Flanigen, J. C. Jansen (Eds.) Studies in Surface Science and Catalysis, Elsevier, 359 (1991).
P. M. Pereira, B. F. Ferreira, N. P. Oliveira, E. J. Nassar, K. J. Ciuffi, M. A. Vicente, R. Trujillano, V. Rives, A. Gil, S. Korili and E. H. De Faria, Appl. Sci., 8, 608 (2018).
H. Awala, E. Leite, L. Saint-Marcel, G. Clet, R. Retoux, I. Naydenova and S. Mintova, New J. Chem., 40, 4277 (2016).
L. Campbell, A. Chimedtsogzol and A. Dyer, Mineralogical Magazine, 70, 361 (2006).
L. S. Campbell, J. Charnock, A. Dyer, S. Hillier, S. Chenery, F. Stoppa, C. M. B. Henderson, R. Walcott and M. Rumsey, Mineralogical Magazine, 80, 781 (2018).
C. A. Başar, J. Hazard. Mater., 135, 232 (2006).
A.-A. Peláez-Cid, A.-M. Herrera-González, M. Salazar-Villanueva and A. Bautista-Hernández, J. Environ. Manage., 181, 269 (2016).
S. Azizian, M. Haerifar and H. Bashiri, Chem. Eng. J., 146, 36 (2009).
C. Djilani, R. Zaghdoudi, F. Djazi, B. Bouchekima, A. Lallam, A. Modarressi and M. Rogalski, J. Taiwan Inst. Chem. Engineers, 53, 112 (2015).
K. D. Belaid, S. Kacha, M. Kameche and Z. Derriche, J. Environ. Chem. Eng., 1, 496 (2013).
D. A. Giannakoudakis, G. Z. Kyzas, A. Avranas and N. K. Lazaridis, J. Mol. Liq., 213, 381 (2016).
Ö. Yavuz and A. H. Aydin, Polish J. Environ. Studies, 15, 155 (2006).
S. M. Mousavi, D. Salari, A. Niaei, P. Nakhostin Panahi and S. Shafiei, Environ. Technol., 35, 581 (2014).
S. M. Mousavi and P. N. Panahi, J. Taiwan Inst. Chem. Eng., 69, 68 (2016).
Sh. Karimifard and M. R. Alavi Moghaddam, Process Saf. Environ. Prot., 99, 20 (2016).
M. K. Satapathy and P. Das, J. Environ. Chem. Eng., 2, 708 (2014).
A. Asfaram, M. Ghaedi, A. Goudarzi and M. Rajabi, Dalton Trans., 44, 14707 (2015).
V. Hernández-Montoya, M. A. Pérez-Cruz, D. I. Mendoza-Castillo, M. R. Moreno-Virgen and A. Bonilla-Petriciolet, J. Environ. Manage., 116, 213 (2013).
N. S. Flores-López, J. Castro-Rosas, R. Ramírez-Bon, A. Mendoza-Córdova, E. Larios-Rodríguez and M. Flores-Acosta, J. Mol. Struct., 1028, 110 (2012).
E. A. Dila, M. Ghaedi and A. Asfaram, Ultrason. Sonochem., 34, 792 (2017).
Y. Xue, H. Hou and S. Zhu, Chem. Eng. J., 147, 272 (2009).
N. Danesh, M. Hosseini, M. Ghorbani and A. Marjani, Synth. Met., 220, 508 (2016).
I. Langmuir, J. Am. Chem. Soci., 38, 2221 (1916).
H. Freundlich, Zeitschrift für Physikalische Chemie, 57U, 385 (1907).
M. J. Temkin and V. Pyzhev, Acta Physicochimica U.R.S.S., 12, 327 (1940).
J. Febrianto, A. N. Kosasih, J. Sunarso, Y.-H. Ju, N. Indraswati and S. Ismadji, J. Hazard. Mater., 162, 616 (2009).
G. Alberti, V. Amendola, M. Pesavento and R. Biesuz, Coord. Chem. Rev., 256, 28 (2012).
K. Y. Foo and B. H. Hameed, Chem. Eng. J., 156, 2 (2010).
H. Bashiri and S. Eris, Chem. Eng. Commun., 203, 628 (2016).
A. M. K. P. Singh, S. Sinha and P. Ojha, J. Hazard. Mater., 150, 626 (2008).
T. C. R. Bertolini, J. C. Izidoro, C. P. Magdalena and D. A. Fungaro, Orbital: Electron. J. Chem., 5, 179 (2013).
E. A. Dila, M. Ghaedi, A. Ghaedi, A. Asfaram, M. Jamshidi and M. K. Purkai, J. Taiwan Inst. Chem. Eng., 59, 210 (2015).
O. S. Amodu, T. V. Ojumu, S. K. Ntwampe and O. S. Ayanda, J. Encapsulation Adsorpt. Sci., 5, 191 (2015).
S. Senthilkumaar, P. Kalaamani and C. V. Subburaam, J. Hazard. Mater., 136, 800 (2006).
B. K. Nandi, A. Goswami, A. K. Das, B. Mondal and M. K. Purkait, Sep. Sci. Technol., 43, 1382 (2008).
H. Yang, D. Zhou, Z. Chang and L. Zhang, Desalin. Water Treat., 52, 6113 (2014).
T. S. Anirudhan, P. S. Suchithra and P. G. Radhakrishnan, Appl. Clay Sci., 43, 336 (2009).
Z. Chen, T. Wang, X. Jin, Z. Chen, M. Megharaj and R. Naidu, J. Colloid Interface Sci., 398, 59 (2013).
S. Hamidzadeh, M. Torabbeigi and S. J. Shahtaheri, J. Environ. Health Sci. Eng., 13, 8 (2015).
P. Rai, R. K. Gautam, S. Banerjee, V. Rawat and M. C. Chattopadhyaya, J. Environ. Chem. Eng., 3, 2281 (2015).
J. P. Simonin, Chem. Eng. J., 300, 254 (2016).
S. Lagergren, Kungliga Svenska Vetenskapsakademiens. Handlingar, 24, 1 (1898).
Y. L. Kang, S. K. S. Toh, P. Monash, S. Ibrahim and P. Saravanan, Asia-Pacific J. Chem. Eng., 8, 811 (2013).
S. Çoruh, F. Geyikçi and O. Nuri Ergun, Environ. Technol., 32, 1183 (2011).
X. Yang and B. Al-Duri, J. Colloid Interface Sci., 287, 25 (2005).
S. Azizian and H. Bashiri, Langmuir, 24, 11669 (2008).
S. Azizian, J. Colloid Interface Sci., 276, 47 (2004).
H. Bashiri and A. Hassani Javanmardi, Chem. Phys. Lett., 671, 1 (2017).
H. Bashiri, Chem. Phys. Lett., 575, 101 (2013).
A. W. Marczewski, Langmuir, 26, 15229 (2010).
M. Haerifar and S. Azizian, J. Phys. Chem. C, 116, 13111 (2012).
H. N. Tran, S.-J. You and H.-P. Chao, J. Environ. Chem. Eng., 4, 2671 (2016).
L. Tagami, O. Santos, E. F. Sousa-Aguiar, P. A. Arroyo and M. Barros, Acta Scientiarum, 23, 1351 (2001).
M. Ishaq, F. Javed, I. Amad and H. Ullah, J. Chem. Chem. Eng., 35, 97 (2016).
Acknowledgement
The authors are grateful to University of Kashan for supporting this work by Grant No. (682211/2).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sarabadan, M., Bashiri, H. & Mousavi, S.M. Removal of crystal violet dye by an efficient and low cost adsorbent: Modeling, kinetic, equilibrium and thermodynamic studies. Korean J. Chem. Eng. 36, 1575–1586 (2019). https://doi.org/10.1007/s11814-019-0356-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11814-019-0356-1