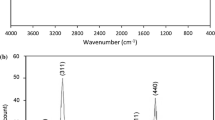
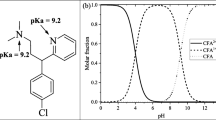
Abstract
Magnetic activated carbon (MAC) was prepared by co-precipitation. These particles had attractive adsorption capacity and could be easily separated from aqueous. MAC was used as adsorbent to remove p-chlorophenol (p-CP) and p-nitrophenol (p-NP) from solution in single and binary systems. In a single system, the equilibrium time was 60 min, the best initial pH was 3-8 and 3-6 for p-CP or p-NP adsorption, respectively. The existence of salt ions had little influence on the adsorption process, while surfactant had negative influence. The adsorption quantity from experiments was up to 97.3 mg·g−1 for p-CP and 116 mg·g−1 for p-NP at 293 K, respectively. Freundlich model and pseudo-second-order kinetic model fitted well the adsorption behavior. Thermodynamic parameters were calculated and the results showed that the process was spontaneous, exothermic and entropy production in nature. In addition, p-CP or p-NP-loaded MAC could be well reused by 0.01 mol·L−1 sodium hydroxide solution as regeneration agent. Kinetic process of desorption was fitted best by pseudo-second-order kinetic model. Results from the binary system showed that competitive adsorption existed during the process, and p-NP adsorption on MAC was easier than p-CP. Freundlich model well fitted the adsorption behavior in the binary system. Hydrogen-bonding, electron donor-acceptor and π-π interactions may be the main mechanisms of adsorption. MAC proved to be an excellent adsorbent for the removal of p-CP and p-NP from solution.
Similar content being viewed by others
References
M. Anbia and S. Khoshbooei, J. Nanostruct. Chem, 5, 139 (2015).
B. Koubaissy, J. Toufaily, M. El-murr, T. Hamieh, P. Magnoux and G. Joly, Phys. Procedia, 21, 220 (2011).
L. R. Rad, I. Haririan and F. Divsar, Spectrochim. Acta A, 136, 423 (2015).
C. A. Acosta, C. Pasquali, G. Paniagua, R. M. Garcinuño and P. F. Hernando, Environ. Pollut., 236, 265 (2018).
C. Borras, T. Laredo and B. R. Scharifker, Electrochim. Acta, 48, 2775 (2003).
H. Ozaki and H. Li, Water Res., 36, 123 (2002).
Y Li and K. C. Loh, J. Appl. Polym. Sci., 105, 1732 (2010).
S. H. Lin and C. S. Wang, J. Hazard. Mater., 90, 205 (2002).
C. Sheng, X. M. Zheng, K. Fei, G. H. Wu, L. L. Luo, G. Yong H. L. Liu, W Ying, H. X. Yu and Z. G. Zou, Mater. Chem. Phys., 129, 1184 (2011).
J. Wu and H. Q. Yu, J. Hazard. Mater., 137, 498 (2006).
J. Su, H. F. Lin, Q. P. Wang, Z. M. Xie and Z. L. Chen, Desalination, 269, 163 (2011).
M. Salman, M. Athar and U. Farooq, Rev. Environ. Sci. Bio., 14, 211 (2015).
J. L. Wang and C. Chen, Biotechnol. Adv., 17, 195 (2009).
A. Bhatnagar, W. Hogland, M. Marques and M. Sillanpää, Chem. Eng. J., 219, 499 (2013).
P. Cañizares, M. Carmona, O. Baraza, A. Delgado and M. A. Rodrigo, J. Hazard. Mater., 131, 243 (2006).
N. Yang, S. M. Zhu, D. Zhang and S. Xu, Mater. Lett., 62, 645 (2008).
S. Singh, Res. J. Chem. Sci., 6, 361 (2015).
S. Han, F. Zhao, J. Sun, B. Wang, R. Y. Wei and S. Q. Yan, J. Magn. Magn. Mater., 341, 133 (2013).
Y. C. Rong, H. Li, L. H. Xiao, Q. Wang, Y. Y. Hu, S. S. Zhang and R. P. Han, Desalin. Water. Treat, 106, 273 (2018).
B. Kakavandi, M. Jahangiri-rad, M. Rafiee, A. R. Esfahani and A. A. Babaei, Micropor. Mesopor. Mater., 231, 192 (2016).
V. L. Lassalle, R. D. Zysler and M. L. Ferreira, Mater. Chem. Phys., 130, 624 (2011).
L. C. A. Oliveira, R. Rios, J. D. Fabris, V. Garg, K. Sapag and R. M. Lago, Carbon, 40, 2177 (2002).
A. Tóth, A. Töröcsik, E. Tombácz and K. László, J. Colloid Interface Sci., 387, 244 (2012).
Q. M. Wei and T. Nakato, Micropor. Mesopor. Mater., 96, 84 (2006).
E. F. Mohamed, C. Andriantsiferana, A. M. Wilhelm and H. Delmas, Environ. Technol., 32, 1325 (2011).
Y. B. Jiao, D. L. Han, Y. Z. Lu, Y. C. Rong, L. Y. Fang, Y. L. Liu and R. P. Han, Desalin. Water. Treat., 77, 247 (2017).
T. Zhou, L. Y. Fang, X. W. Wang, M. Y. Han, S. S. Zhang and R. P. Han, Desalin. Water Treat., 70, 294 (2017).
I. K. Battisha, H. H. Afify and M. Ibrahim, J. Magn. Magn. Mater., 306, 211 (2006).
J. Y. Song, W. H. Zou, Y. Y. Bian, F. Y. Su and R. P. Han, Desalination, 265, 119 (2011).
R. D. Zhang, J. H. Zhang, X. N. Zhang, C. C. Dou and R. P. Han, J. Taiwan Inst. Chem. E., 45, 2578 (2014).
N. Li, J. Chen and Y. P. Shi, Anal. Chim. Acta, 949, 23 (2017).
G. Mihoc, R. Ianoç and C. Păcurariu, Water Sci. Technol., 69, 385 (2014).
B. Zhang, F. Li, T. Wu, D. J. Sun and Y. J. Li, Colloids Surf, A., 464, 78 (2015).
S. K. Srivastava and R. Tyagi, Water Res., 29, 483 (1995).
F. Zhang, Z. Wei, W. N. Zhang and H. Y. Cui, Spectrochim. Acta A, 182, 116 (2017).
Y. S. Ho and G. Mckay, Process Biochem., 34, 451 (1999).
C.W. Cheung, J.F. Porterand G. Mckay, Sep. Purif. Technol., 19, 55 (2000).
B. H. Hameed and A. A. Rahman, J. Hazard. Mater., 160, 576 (2008).
A. E. Ofomaja and E. I. Unuabonah, Carbohydr. Polym., 83, 1192 (2011).
A. E. Vasu, E-J. Chem, 5, 224 (2012).
I. A. Bello, M. A. Oladipo, A. A. Giwa and D. O. Adeoye, Int. J. Basic Appl. Sci., 2, 79 (2013).
J. M. Li, X. G. Meng, C. W. Hu and J. Du, Bioresour. Technol., 100, 1168 (2009).
H. A. Arafat, M. Franz and N. G. Pinto, Langmuir, 15, 5997 (1999).
K. Yang, Q. Jing, W. Wu, L. Zhu and B. Xing, Environ. Sci. Technol., 44, 681 (2010).
M. Ahmaruzzaman and D. K. Sharma, J. Colloid Interface Sci., 287, 14 (2005).
K. A. Halhouli, N. A. Darwish and Y. R. Y. Al-Jahmany, Sep. Sci. Technol., 32, 3027 (1997).
G. Crini, Dyes Pigm., 77, 415 (2008).
R. P. Han, Y. F. Wang, P. Han, J. Shi, J. Yang and Y. S. Lu, J. Hazard. Mater., 137, 550 (2006).
M. Chabani, A. Amraneb and A. Bensmaili, Chem. Eng. J, 125, 111 (2006).
T. S. Anirudhan and M. Ramachandran, J. Water Process Eng., 1, 46 (2014).
C. Y. Chang, W. T. T. Sai, C. H. Ing and C. H. Chang, J. Colloid Interface Sci., 260, 273 (2003).
Y. Fan, R. F. Yang, Z. M. Lei, N. Liu, J. L. Lv, S. R. Zhai, B. Zhai and L. Wang, Korean J. Chem. Eng., 33, 1416 (2016).
T. Zhou, W. Z. Lu, L. F. Liu, H. M. Zhu, Y. B. Jiao, S. S. Zhang and R. P. Han, J. Mol. Liq., 211, 909 (2015).
M. L. Nguyen and R. S. Juang, Biotechnol. Bioproc. E., 20, 614 (2015).
J. M. Chern and Y. W. Chien, Water Res., 37, 2347 (2003).
S. R. Ha and S. Vinitnantharat, Environ. Technol., 21, 387 (2000).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rong, Y., Han, R. Adsorption of p-chlorophenol and p-nitrophenol in single and binary systems from solution using magnetic activated carbon. Korean J. Chem. Eng. 36, 942–953 (2019). https://doi.org/10.1007/s11814-019-0267-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11814-019-0267-1