Abstract
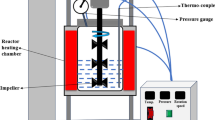
Hydrochars derived from golden shower pod (GSH), coconut shell (CCH), and orange peel (OPH) were synthesized and applied to remove methylene green (MG5). The results indicated that the hydrochars possessed low specific surface areas (6.65-14.7m2/g), but abundant oxygen functionalities (1.69-2.12mmol/g). The hydrochars exhibited cellular and spherical morphologies. Adsorption was strongly dependent on the solution pH (2-10) and ionic strength (0-0.5M NaCl). Equilibrium can be quickly established in the kinetic study (60-120 min). The maximum Langmuir adsorption capacities at 30 °C followed the order GSH (59.6mg/g)>CCH (32.7mg/g)>OPH (15.6mg/g)> commercial glucose-prepared hydrochar (12.6mg/g). The dye adsorption efficiency was determined by the concentrations of oxygen-containing functionalities on the hydrochar surface. The adsorption process occurred spontaneously (− ΔGo) and exothermically (−ΔHo). Desorption studies confirmed the reversible adsorption process. Oxygenation of the hydrochar surface through a hydrothermal process with acrylic acid contributed to increasing MG5 adsorption and identifying the negligible role of π-π interaction to the adsorption process. The analysis of Fourier transform infrared spectrometry demonstrated that the aromatic C=C peak did not significantly decrease in intensity or shift toward higher/lower wavenumbers after adsorption, which further confirms the insignificant contribution of π-π interaction. Electrostatic attraction played a major role in adsorption mechanisms, while minor contributions were accounted for hydrogen bonding and n-π interactions. The primary adsorption mechanisms of MG5 onto hydrochar were similar to biosorbent, but dissimilar to biochar and activated carbon (i.e., π-π interaction and pore filling).
Similar content being viewed by others
References
F.M.D. Chequer, G.A.R. de Oliveira, E.R.A. Ferraz, J.C. Cardoso, M.V.B. Zanoni and D. P. de Oliveira, Eco-friendly Textile Dyeing and Finishing, INTECH Publishers, 151 (2013).
N. P. Raval, P.U. Shah and N.K. Shah, Environ. Sci. Pollut. r., 23, 14810 (2016).
G. Crini, Bioresour. Technol., 97, 1061 (2006).
E. Contreras, L. Sepúlveda and C. Palma, Int. J. Chem. Eng., 2012, 1 (2012).
N. Feng, X. Guo and S. Liang, J. Hazard. Mater., 164, 1286 (2009).
H. N. Tran, S.-J. You and H.-P. Chao, Waste Manage. Res., 34, 129 (2016).
J. S. Cha, S. H. Park, S. C. Jung, C. Ryu, J. K. Jeon, M. C. Shin and Y. K. Park, J. Ind. Eng. Chem., 40, 1 (2016).
H. N. Tran, Y.-F. Wang, S.-J. You and H.-P. Chao, Trans. IChemE Process Saf. Environ. Prot., (2017), DOI:10.1016/j.psep.2017.02.010.
H. N. Tran, S.-J. You and H.-P. Chao, J. Environ. Manage., 188, 322 (2017).
J.A. Libra, K. S. Ro, C. Kammann, A. Funke, N.D. Berge, Y. Neubauer, M.M. Titirici, C. Fühner, O. Bens, J. Kern and K.H. Emmerich, Biofuels, 2, 71 (2011).
A. Jain, R. Balasubramanian and M. P. Srinivasan, Chem. Eng. J., 283, 789 (2016).
H.N. Tran, S.-J. You and H.-P. Chao, Adsorpt. Sci. Technol., (2017), DOI:10.1177/0263617416684837.
A. Funke and F. Ziegler, Biofuel. Bioprod. Bior., 4, 160 (2010).
H.N. Tran, F.-C. Huang, C.-K. Lee and H.-P. Chao, Green Process. Synth., (2017), DOI:10.1515/gps-2016-0178.
S. L. Goertzen, K.D. Thériault, A. M. Oickle, A. C. Tarasuk and H. A. Andreas, Carbon, 48, 1252 (2010).
M. Sevilla and A.B. Fuertes, Chem. Eur. J., 15, 4195 (2009).
M. Sevilla and A.B. Fuertes, Carbon, 47, 2281 (2009).
M. Sevilla, J. A. Maciá-Agulló and A.B. Fuertes, Biomass Bioenergy, 35, 3152 (2011).
M. Sevilla, A. B. Fuertes and R. Mokaya, Energy Environ. Sci., 4, 1400 (2011).
M. Dogan, H. Abak and M. Alkan, J. Hazard. Mater., 164, 172 (2009).
Y. Guo, S. Yang, W. Fu, J. Qi, R. Li, Z. Wang and H. Xu, Dyes Pigments, 56, 219 (2003).
S. Lagergren, Ksver. Veterskapsakad. Handl., 24, 1 (1898).
G. Blanchard, M. Maunaye and G. Martin, Water Res., 18, 1501 (1984).
S. H. Chien and W.R. Clayton, Soil Sci. Soc. Am. J., 44, 265 (1980).
H.N. Tran, S.-J. You and H.-P. Chao, Chem. Eng. Commun., (2017), DOI:10.1016/j.watres.2017.04.014.
H.N. Tran, S.-J. You and H.-P. Chao, J. Environ. Chem. Eng., 4, 2671 (2016).
J.A. Mattson, H. B. Mark, M.D. Malbin, W. J. Weber and J. C. Crittenden, J. Colloid Interface Sci., 31, 116 (1969).
B. Xing, W. B. McGill, M. J. Dudas, Y. Maham and L. Hepler, Environ. Sci. Technol., 28, 466 (1994).
L.R. Radovic, C. Moreno-Castilla and J. Rivera-Utrilla, Chemistry and physics of carbon, Marcel Dekker, Inc., New York, 27, 227 (2000).
R.W. Coughlin and F. S. Ezra, Environ. Sci. Technol., 2, 291 (1968).
R. S. Blackburn, Environ. Sci. Technol., 38, 4905 (2004).
M. A. Al-Ghouti, M.A.M. Khraisheh, S. J. Allen and M.N. Ahmad, J. Environ. Manage., 69, 229 (2003).
M.D. Huff and J.W. Lee, J. Environ. Manage., 165, 17 (2016).
R. Demir-Cakan, N. Baccile, M. Antonietti and M. M. Titirici, Chem. Mater., 21, 484 (2009).
J. Xu, L. Wang and Y. Zhu, Langmuir, 28, 8418 (2012).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Tran, H.N., You, SJ. & Chao, HP. Insight into adsorption mechanism of cationic dye onto agricultural residues-derived hydrochars: Negligible role of π-π interaction. Korean J. Chem. Eng. 34, 1708–1720 (2017). https://doi.org/10.1007/s11814-017-0056-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11814-017-0056-7