Abstract
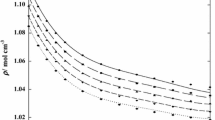
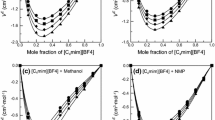
Densities of nine binary solutions made of one of three 1-butyl-3-methylimidazolium ([bmim]) halides with water, methanol, or ethanol were measured at atmospheric pressure. The compositions of an ionic liquid ([bmim]Cl, [bmim]Br, or [bmim]I) were increased up to 0.4 as a mole fraction at a given temperature within a range of 293.15 to 318.15 K. The measured values were correlated by a quadratic equation to obtain a temperature dependency of the respective systems. Furthermore, the equation was used to obtain the volume expansivity, which would be used for a pressure-volume-temperature behavior of a condensed phase. The apparent molar volumes were also calculated from the experimental data. The remarkable distinction of the volumetric property behavior between aqueous and nonaqueous solutions was found and attributed to strong ion–solvent interactions in the aqueous systems.
Similar content being viewed by others
References
B. Kirchner Ed., Ionic Liquids, Springer, New York (2010).
J. L. Anthony, E. J. Maginn and J. F. Brennecke, J. Phys. Chem. B, 105, 10942 (2001).
J. Dupoint, R. F. de Souza and P. A. Z. Suarez, Chem. Rev., 102, 3667 (2002).
M. Hayyan, F. S. Mjalli, M. A. Hashim, I. M. AlNashef and T. X. Mei, J. Ind. Eng. Chem., 19, 106 (2013).
D. Betz, P. Altmann, M. Cokoja, W. A. Herrmann and F. E. Kühn, Coordin. Chem. Rev., 255, 1518 (2011).
D. Tian, Y. Han, C. Lu, X. Zhang and G. Yuan, Carbohydr. Polym., 113, 83 (2014).
W.-J. Chen, W.-T. Lou, C.-Y. Yu, H. Wu, M.-H. Zong and T. J. Smith, J. Biotechnol., 162, 183 (2012).
K.-S. Kim, S.-Y. Park, S. Choi and H. Lee, J. Power Sources, 155, 385 (2006).
B. S. Shin, E. S. Kim, S. K. Kwak, J. S. Lim, K.-S. Kim and J. W. Kang, Fluid Phase Equilib., 382, 270 (2014).
M. Jayakumar, K. A. Venkatesan and T. G. Srinivasan, Electrochim. Acta, 53, 2794 (2008).
S. Baj, T. Krawczyk, A. Dabrowska, A. Siewniak and A. Sobolewski, Korean J. Chem. Eng., 32, 2295 (2015).
K.-S. Kim, B.-K. Shin, H. Lee and F. Ziegler, Fluid Phase Equilib., 218, 215 (2004).
G. Fan, C. Liao, T. Fang, M. Wang and G. Song, Fuel Process. Technol., 116, 142 (2013).
X. He, B. Hou, C. Li, Q. Zhu, Y. Jiang and L. Wu, Electrochim Acta, 130, 245 (2014).
P. Bonhte, A. P. Dias, N. Papageorgiou, K. Kalyanasundaram and M. Gratzel, Inorg. Chem., 35, 1168 (1996).
U. Domanska and A. Marciniak, J. Chem. Eng. Data, 48, 451 (2003).
U. Domanska, E. Bogel-Lukasik and R. Bogel-Lukasik, J. Phys. Chem. B, 107, 1858 (2003).
R. P. Swatloski, A. E. Visser, W. M. Reichert, G. A. Broker, L. M. Farina, J. D. Holbrey and R. D. Rogers, Green Chem., 4, 81 (2002).
T. M. Letcher, N. Deenadayalu, B. Soko, D. Ramjugernath and P. K. Naicker, J. Chem. Eng. Data, 48, 904 (2003).
J. G. Huddleston, A. E. Visser, W. M. Reichert, H. D. Willauer, G. A. Broker and R. D. Rogers, Green Chem., 3, 156 (2001).
Q. Yang, H. Zhang, B. Su, Y. Yang, Q. Ren and H. Xing, J. Chem. Eng. Data, 55, 1750 (2010).
B. Lal, M. Sahin and E. Ayranci, J. Chem. Thermodyn., 54, 142 (2012).
D. Matkowska and T. Hofman, J. Mol. Liq., 177, 301 (2013).
R. Sadeghi, H. Shekaari and R. Hosseini, J. Chem. Thermodyn., 41, 273 (2009).
N. V. Sastry, N. M. Vaghela and P. M. Macwan, J. Mol. Liq., 180, 12 (2013).
M. T. Zafarani-Moattar and H. Shekaari, J. Chem. Thermodyn., 37, 1029 (2005).
W. Wen and S. Saito, J. Phys. Chem., 68, 2639 (1964).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Park, B.H. Volumetric properties of binary mixtures of 1-butyl-3-methylimidazolium halides with water, methanol or ethanol at 293.15 to 318.15 K. Korean J. Chem. Eng. 33, 2191–2204 (2016). https://doi.org/10.1007/s11814-016-0053-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11814-016-0053-2