Abstract
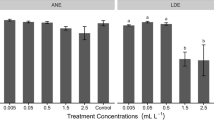
In this study, we investigated the effects of different concentrations of 5-azacytidine (AZA), a DNA methyltransferase inhibitor, on the growth, antioxidant activities and germination of pellicle cysts of Scrippsiella acuminata. The purpose of this study is to understand the toxic effects of AZA on marine microalgae, and to demonstrate the effect of DNA methyltransferase inhibitors on the germination of pellicle cysts. Results showed that AZA inhibited the growth of S. acuminata significantly, and displaced a clear dose-dependent inhibition trend with the 96h EC50 of 146.77µmolL−1 (35.84 mg L−1). Pellicle cysts of S. acuminata were less sensitive to AZA than the vegetative cells, and the EC50 value of AZA to the germination of pellicle cysts of S. acuminata was 8.08mmolL−1 (1.97g L−1). After exposed to AZA, the antioxidant activities in S. acuminata responded rapidly and significantly. Among them, soluble protein and superoxide dismutase (SOD) were more sensitive to AZA, and significant promotions occurred after exposed to 10 µmolL−1 AZA for 24 h. Meanwhile, malondialdehyde (MDA) contents in algal cells did not change significantly after exposed to low concentrations of AZA, but increased firstly and then decreased under high concentration of AZA. The glutathione (GSH) levels in S. acuminata increased significantly under high concentrations of AZA, and remained unchanged at low concentrations of AZA. The results suggested that the enhanced protein level and SOD activity of S. acuminata eliminated reactive oxygen species (ROS) to a certain extent, and thus protected algal cells against damages of ROS caused by AZA.
Similar content being viewed by others
References
Al-Rashed, S. A., Ibrahim, M. M., El-Gaaly, G. A., Al-Shehri, S., and Mostafa, A., 2016. Evaluation of radical scavenging system in two microalgae in response to interactive stresses of UV-B radiation and nitrogen starvation. Saudi Journal of Biological Sciences, 23(6): 706–712, DOI: https://doi.org/10.1016/j.sjbs.2016.06.010.
Alscher, R. G., Erturk, N., and Heath, L. S., 2002. Role of superoxide dismutases (SODs) in controlling oxidative stress in plants. Journal of Experimental Botany, 53(372): 1331–1341, DOI: https://doi.org/10.1093/jexbot/53.372.1331.
Annereau, M., Desmaris, R., Micol, J. B., Lazarovici, J., Chenallier, C., Saada, V., et al., 2014. Results of treatment with 5-aza-cytidine in patients treated for myelodysplastic syndrome or secondary acute myeloid leukemia associated with a concomitant active cancer. Journal of Clinical Oncology, 32(15S): e18029, DOI: https://doi.org/10.1200/jco.2014.32.15_suppl.e18029.
Bacova, R., Klejdus, B., Ryant, P., Cernei, N., Adam, V., and Huska, D., 2019. The effects of 5-azacytidine and cadmium on global 5-methylcytosine content and secondary metabolites in the freshwater microalgae Chlamydomonas reinhardtii and Sce-nedesmus quadricauda. Journal of Phycology, 55(2): 329–342, DOI: https://doi.org/10.1111/jpy.12819.
Betekhtin, A., Milewska-Hendel, A., Chajec, L., Rojek, M., Nowak, K., Kwasniewska, J., et al., 2018. 5-Azacitidine induces cell death in a tissue culture of Brachypodium distachyon. International Journal of Molecular Sciences, 19(6): 1806, DOI: https://doi.org/10.3390/ijms19061806.
Bravo, I., and Figueroa, R. I., 2014. Towards an ecological understanding of dinoflagellate cyst functions. Microorganisms, 2(1): 11–32, DOI: https://doi.org/10.3390/microorganisms2010011.
Bravo, I., Figueroa, R. I., Garcés, E., Fraga, S., and Massanet, A., 2010. The intricacies of dinoflagellate pellicle cysts: The example of Alexandrium minutum cysts from a bloom-recurrent area (Bay of Baiona, NW Spain). Deep Sea Research Part II: Topical Studies in Oceanography, 57(3–4): 166–174, DOI: https://doi.org/10.1016/j.dsr2.2009.09.003.
Chen, R. Z., Chen, X. H., Huo, W., Zheng, S. Z., Lin, Y. L., and Lai, Z. X., 2021. Transcriptome analysis of azacitidine (5-AzaC)-treatment affecting the development of early somatic embryogenesis in longan. The Journal of Horticultural Science and Biotechnology, 96(3): 311–323, DOI: https://doi.org/10.1080/14620316.2020.1847695.
Cheng, Y. H., Peng, X. Y., Yu, Y. C., Sun, Z. Y., and Han, L., 2019. The Effects of DNA methylation inhibition on flower development in the dioecious plant Salix viminalis. Forests, 10(2): 173, DOI: https://doi.org/10.3390/f10020173.
Christman, J. K., 2002. 5-azacytidine and 5-aza-2’-deoxycytidine as inhibitors of DNA methylation: Mechanistic studies and their implications for cancer therapy. Oncogene, 21(35): 5483–5495, DOI: https://doi.org/10.1038/sj.onc.1205699.
Fan, X., Han, W. T., Teng, L. H., Jiang, P., Zhang, X. W., Xu, D., et al., 2020. Single-base methylome profiling of the giant kelp Saccharina japonica reveals significant differences in DNA methylation to microalgae and plants. New Phytologist, 225(1): 234–249, DOI: https://doi.org/10.1111/nph.16125.
Feng, T. Y., and Chiang, K. S., 1984. The persistence of maternal inheritance in Chlamydomonas despite hypomethylation of chloroplast DNA induced by inhibitors. Proceedings of the National Academy of Sciences. USA, 81(11): 3438–3442, DOI: https://doi.org/10.1073/pnas.81.11.3438.
Gao, Q. T., Wong, Y. S., and Tam, N. F. Y., 2017. Antioxidant responses of different microalgal species to nonylphenol-induced oxidative stress. Journal of Applied Phycology, 29(3): 1317–1329, DOI: https://doi.org/10.1007/s10811-017-1065-y.
Garcés, E., Masó, M., and Camp, J., 2002. Role of temporary cysts in the population dynamics of Alexandrium taylori (Dinophyceae). Journal of Plankton Research, 24(7): 681–686, DOI: https://doi.org/10.1093/plankt/24.7.681.
Guillard, R. R. L., 1975. Culture of phytoplankton for feeding marine invertebrates. In: Culture of Marine Invertebrate Animals. Smith, W. L., and Chanley, M. H., eds., Springer, Boston, MA, 29–60.
Guo, X., Wang, Z. H., Liu, L., and Li, Y., 2021. Transcriptome and metabolome analyses of cold and darkness-induced pellicle cysts of Scrippsiella trochoidea. BMC Genomics, 22(1): 526, DOI: https://doi.org/10.1186/s12864-021-07840-7.
Ho, P., Kong, K. F., Chan, Y. H., Tsang, J. S. H., and Wong, J. T. Y., 2007. An unusual S-adenosylmethionine synthetase gene from dinoflagellate is methylated. BMC Molecular Biology, 8(1): 87, DOI: https://doi.org/10.1186/1471-2199-8-87.
Li, M. L., Zeng, G. W., and Zhu, Z. J., 2003. Analysis of effects of 5-azficytidine on promoting flowering in non-heading Chinese cabbage. Journal of Zhejiang University (Agriculture and Life Sciences), 29(3): 287–290 (in Chinese with English abstract).
Li, X. F., Wang, W., Zhang, X., and Wu, Y., 2022. Azacitidine and donor lymphocyte infusion for patients with relapsed acute myeloid leukemia and myelodysplastic syndromes after allogeneic hematopoietic stem cell transplantation: A meta-analysis. Frontiers in Oncology, 12: 949534, DOI: https://doi.org/10.3389/fonc.2022.949534.
Li, X. F., Xia, Z. Y., Tang, J. Q., Wu, J. H., Tong, J., Li, M. J., et al., 2017. Identification and biological evaluation of secondary metabolites from marine derived fungi–Aspergillus sp. SCSIOW3, cultivated in the presence of epigenetic modifying agents. Molecules, 22(8): 1302, DOI: https://doi.org/10.3390/molecules22081302.
Lin, S. J., 2011. Genomic understanding of dinoflagellates. Research in Microbiology, 162(6): 551–269, DOI: https://doi.org/10.1016/j.resmic.2011.04.006.
Lindeman, L. C., Thaulow, J., Song, Y., Kamstra, J. H., Xie, L., Asselman, J., et al., 2019. Epigenetic, transcriptional and phenotypic responses in two generations of Daphnia magna exposed to the DNA methylation inhibitor 5-azacytidine. Environmental Epigenetics, 5(3): 1–12, DOI: https://doi.org/10.1093/eep/dvz016.
Lohuis, M. R., and Miller, D. J., 1998. Light-regulated transcription of genes encoding peridinin chlorophyll a proteins and the major intrinsic light-harvesting complex proteins in the dinoflagellate Amphidinium carterae Hulburt (Dinophycae): Changes in cytosine methylation accompany photoadaptation. Plant Physiology, 117(1): 189–196, DOI: https://doi.org/10.1104/pp.117.1.189.
Lopez, D., Hamaji, T., Kropat, J., Hoff, D. P., Morselli, M., Rubbi, L., et al., 2015. Dynamic changes in the transcriptome and methylome of Chlamydomonas reinhardtii throughout its life cycle. Plant Physiology, 169(4): 2730–2743, DOI: https://doi.org/10.1104/pp.15.00861.
Lu, Y. C., Feng, S. J., Zhang, J. J., Luo, F., Zhang, S., and Yang, H., 2016. Genome-wide identification of DNA methylation provides insights into the association of gene expression in rice exposed to pesticide atrazine. Scientific Report, 6: 18985, DOI: https://doi.org/10.1038/srep18985.
Mishra, S., Srivastava, S., Tripathi, R. D., Kumar, R., Seth, C. S., and Gupta, D. K., 2006. Lead detoxification by coontail (Ceratophyllum demersum L.) involves induction of phytochelatins and antioxidant system in response to its accumulation. Chemosphere, 65(6): 1027–1039, DOI: https://doi.org/10.1016/j.chemosphere.2006.03.033.
Mittler, R., 2002. Oxidative stress, antioxidants and stress tolerance. Trends in Plant Science, 7(9): 405–410, DOI: 10.1016/S1360–1385(02)02312-9.
Nowicka, A., Juzon, K., Krzewska, M., Dziurka, M., Dubas, E., Kopec, P., et al., 2019. Chemically-induced DNA de-methylation alters the effectiveness of microspore embryogenesis in triticale. Plant Science, 287: 110189, DOI: https://doi.org/10.1016/j.plantsci.2019.110189.
Ogneva, Z. V., Suprun, A. R., Dubrovina, A. S., and Kiselev, K. V., 2019. Effect of 5-azacytidine induced DNA demethylation on abiotic stress tolerance in Arabidopsis thaliana. Plant Protection Science, 55(2): 73–80, DOI: https://doi.org/10.17221/94/2018-PPS.
Olli, K., 2004. Temporary cyst formation of Heterocapsa triquetra (Dinophyceae) in natural populations. Marine Biology, 145(1): 1–8, DOI: https://doi.org/10.1007/s00227-004-1295-9.
Onda, D. F. L., Lluisma, A. O., and Azanza, R. V., 2014. Development, morphological characteristics and viability of temporary cysts of Pyrodinium bahamense var. compressum (Dinophyceae) in vitro. European Journal of Phycology, 49(3): 265–275, DOI: https://doi.org/10.1080/09670262.2014.915062.
Osorio-Montalvo, P., Sáenz-Carbonell, L., and De-la-Peña, C., 2018. 5-Azacytidine: A promoter of epigenetic changes in the quest to improve plant somatic embryogenesis. International Journal of Molecular Science, 19(10): 3182–3182, DOI: https://doi.org/10.3390/ijms19103182.
Palsamy, P., Bidasee, K. R., and Shinohara, T., 2014. Valproic acid suppresses Nrf2/Keap1 dependent antioxidant protection through induction of endoplasmic reticulum stress and Keap1 promoter DNA demethylation in human lens epithelial cells. Experimental Eye Research, 121: 26–34, DOI: https://doi.org/10.1016/j.exer.2014.01.021.
Powles, S. B., 1984. Photoinhibition of photosynthesis induced by visible-light. Annual Review of Plant Biology, 35(1): 15–44, DOI: https://doi.org/10.1146/annurev.pp.35.060184.000311.
Sano, H., Kamada, I., Youssefian, S., Katsumi, M., and Wabiko, H., 1990. A single treatment of rice seedlings with 5-azacy-tidine induces heritable dwarfism and undermethylation of genomic DNA. Molecular and General Genetics, 220(3): 441–447, DOI: https://doi.org/10.1007/BF00391751.
Sheldon, C. C., Burn, J. E., Perez, P. P., Metzger, J., Edwards, J. A., Peacock, W. J. et al, 1999. The FLF MADS box gene: A repressor of flowering in arabidopsis regulated by vernalization and methylation. The Plant Cell, 11(3): 445–458, DOI: https://doi.org/10.1105/tpc.11.3.445.
Shin, H. H., Li, Z., Yoon, Y. H., Oh, S. J., and Lim, W. A., 2017. Formation and germination of temporary cysts of Cochlodinium polykrikoides Margalef (Dinophyceae) and their ecological role in dense blooms. Harmful Algae, 66: 57–64, DOI: https://doi.org/10.1016/j.hal.2017.05.002.
Stephens, T. G., Gonzalez-Pech, R. A., Cheng, Y. Y., Mohamed, A. R., Burt, D. W., Bhattacharya, D., et al, 2020. Genomes of the dinoflagellate Polarella glacialis encode tandemly repeated single-exon genes with adaptive functions. BMC Biology, 18: 56, DOI: https://doi.org/10.1186/s12915-020-00782-8.
Taşkin, K. M., Özbilen, A., Sezer, F., Hurkan, K., and Gunes, S., 2017. Structure and expression of DNA methyltransferase genes from apomictic and sexual Boechera species. Computational Biology and Chemistry, 67: 15–21, DOI: https://doi.org/10.1016/j.compbiolchem.2016.12.002.
Veluchamy, A., Lin, X., Maumus, F., Rivarola, M., Bhavsar, J., Creasy, T., et al., 2013. Insights into the role of DNA methylation in diatoms by genome-wide profiling in Phaeodactylum tricornutum. Nature Communications, 4: 2091, DOI: https://doi.org/10.1038/ncomms3091.
Wang, Z. C., Nie, L. J., and He, Y. X., 2009. The effect of 5-azacytidine to the DNA methylation and morphogenesis character of chrysanthemum during in vitro growth. Acta Horticulturae Sinica, 36(12): 1783–1790 (in Chinese with English abstract).
Wang, Z. H., Nie, X. P., Yue, W. J., and Li, X., 2011. Physiological responses of three marine microalgae exposed to cyper-methrin. Environmental Toxicology, 27(10): 563–572, DOI: https://doi.org/10.1002/tox.20678.
Wang, Z. H., Qi, Y. Z., and Yang, Y. F., 2007. Cyst formation: An important mechanism for the termination of Scrippsiella trochoidea (Dinophyceae) bloom. Journal of Plankton Research, 29(2): 209–218, DOI: https://doi.org/10.1093/plankt/fbm008.
Yan, T., Li, X., Cao, Y., Wu, B. Y., Wang, J., and Zhang, M. M., 2021. Effects of 5-azacytidine on drought yolerance of rice with high expression of C4-PEPC. Acta Agriculturae Boreali-Sinica, 36(4): 96–107 (in Chinese with English abstract).
Yang, F., Li, L., and Lin, S. J., 2020. Methylation pattern and expression dynamics of methylase and photosystem genes under varying light Intensities in Fugacium kawagutii (Symbio-diniaceae). Journal of Phycology, 56(6): 1738–1747, DOI: https://doi.org/10.1111/jpy.13070-20-045.
Zhang, Y. X., Si, F. H., Wang, Y. Y., Liu, C. Y., Zhang, T., Yuan, Y. C., et al., 2020. Application of 5-azacytidine induces DNA hypomethylation and accelerates dormancy release in buds of tree peony. Plant Physiology and Biochemistry, 147: 91–100, DOI: https://doi.org/10.1016/j.plaphy.2019.12.010.
Zhao, J. G., Tang, T., Zhang, J. N., Guo, H., and Wang, Z. H., 2022. Studies on growth and antioxidant responses of two dinoflagellates species under exposure to decitabine. Asian Journal of Ecotoxicology, 17(3): 468–476 (in Chinese with English abstract).
Zinssmeister, C., Soehner, S., Facher, E., Kirsch, M., Meier, K. J. S., and Gottschling, M., 2011. Catch me if you can: The taxonomic identity of Scrippsiella trochoidea (F. Stein) A.R.Loebl. (Thoracosphaeraceae, Dinophyceae). Systematics and Biodiversity, 9(2): 145–157, DOI: https://doi.org/10.1080/14772000.2011.586071.
Zutshi, S., Choudhary, M., Bharat, N., Abdin, M. Z., and Fatma, T., 2008. Evaluation of antioxidant defense responses to lead stress in Hapalosiphon fontinalis-339. Journal of Phycology, 44(4): 889–896, DOI: https://doi.org/10.1111/j.1529-8817.2008.00542.x.
Acknowledgement
This work was supported by the National Natural Science Foundation of China (No. 42076141).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Wang, Z., Zhang, J., Tang, T. et al. Effects of 5-Azacytidine (AZA) on the Growth, Antioxidant Activities and Germination of Pellicle Cysts of Scrippsiella acuminata (Dinophyceae). J. Ocean Univ. China 22, 1660–1668 (2023). https://doi.org/10.1007/s11802-023-5583-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11802-023-5583-8