Abstract
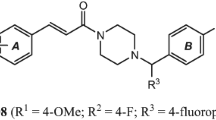
Alkaloids are a class of natural products with a wide range of biological activities. Due to the special living environment, the alkaloids from marine sponges have exhibited different biological activities and promising medical application potential. Neolamellarin A is a marine alkaloid possessing bisaryl-pyrrole structural features. Here, the synthesis of 12 different 3,4-bisaryl-N-alkylated permethylated analogues of neolamellarin A and their outstanding neuroprotective activity in PC12 cells are presented and discussed.
Similar content being viewed by others
References
Ayedi, M. A., Le Bigot, Y., Ammar, H., Abid, S., El Gharbi, R., and Delmas, M., 2013. ChemInform abstract: Synthesis of primary amines by one-pot reductive amination of aldehydes. ChemInform, 43 (44): 2127–2133, DOI: 10.1002/chin.201343053.
Bailly, C., 2014. Lamellarins: A tribe of bioactive marine natural products. In: Outstanding Marine Molecules. LA Barre, S., and Kornprobst, J., eds., Wiley-VCH Verlag GmbH & Co. KGaA, 377–386.
Bharate, J. B., Singh, S., Wani, A., Sharma, S., Joshi, P., Khan, I. A., Kumar, A., Vishwakarma, R. A., and Bharate, S. B., 2015. Discovery of 4-acetyl-3-(4-fluorophenyl)-1-(p-tolyl)-5-methy-lpyrrole as a dual inhibitor of human P-glycoprotein and Staphylococcus aureus Nor A efflux pump. Organic & Biomolecular Chemistry, 19 (13): 5424–5431, DOI: 10.1039/c5ob00246j.
Fan, A. L., Lin, W. H., and Jia, Y. X., 2011. Recent progress in the research on lamellarins and related pyrrole-derived alkaloids from marine organisms. Journal of Chinese Pharmaceutical Sciences, 5 (20): M5, DOI: 10.5246/jcps.2011.05.054.
Fan, H., Peng, J., Hamann, M. T., and Hu, J., 2008. ChemInform abstract: Lamellarins and related pyrrole-derived alkaloids from marine organisms. ChemInform, 18 (39): 264–287, DOI: 10.1002/chin.200818251.
Hao, C., Gao, L., Zhang, Y., Wang, W., Yu, G., Guan, H., Zhang, L., and Li, C., 2015. Acetylated chitosan oligosaccharides act as antagonists against glutamate-induced PC12 cell death via Bcl-2/Bax signal pathway. Marine Drugs, 3 (13): 1267–1289, DOI: 10.3390/md13031267.
He, Y., Lan, Y., Liu, Y., Yu, H., Han, Z., Li, X., and Zhang, L., 2016. Pingyangmycin and bleomycin share the same cytotoxicity pathway. Molecules, 7 (21): DOI: 10.3390/molecules 21070862.
Li, Q., Fan, A., Lu, Z., Cui, Y., Lin, W., and Jia, Y., 2010. One-pot AgOAc-mediated synthesis of polysubstituted pyrroles from primary amines and aldehydes: Application to the total synthesis of purpurone. Organic Letters, 18 (12): 4066–4069, DOI: 10.1021/ol101644g.
Liu, R., Liu, Y., Zhou, Y. D., and Nagle, D. G., 2007. Molecular targeted antitumor agents. 15. Neolamellarins from the marine sponge Dendrilla nigra inhibit hypoxia-inducible factor-1 activation and secreted vascular endothelial growth factor production in breast tumor cells. Journal of Natural Products, 11 (70): 1741–1745, DOI: 10.1021/np070206e.
Mosmann, T., 1983. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. Journal of Immunological Methods, 65 (1-2): 55–63, DOI: 10.1016/0022-1759(83)90303-4.
Plisson, F., Huang, X. C., Zhang, H., Khalil, Z., and Capon, R. J., 2012. Lamellarins as inhibitors of P-glycoprotein-mediated multidrug resistance in a human colon cancer cell line. Chemistry Asian Journal, 7 (7): 1616–1623, DOI: 10.1002/asia.201101049.
Quesada, A. R., Grávalos, M. G., and Puentes, J. F., 1996. Polyaromatic alkaloids from marine invertebrates as cytotoxic compounds and inhibitors of multidrug resistance caused by P-glycoprotein. British Journal of Cancer, 74 (5): 677–682, DOI: 10.1038/bjc.1996.421.
Rosowsky, A., Chen, H., Fu, H., and Queener, S. F., 2003. Synthesis of new 2,4-Diaminopyrido[2,3-d]pyrimidine and 2,4-Diaminopyrrolo[2,3-d]pyrimidine inhibitors of Pneumocystis carinii, Toxoplasma gondii, and Mycobacterium avium dihydrofolate reductase. Bioorganic & Medicinal Chemistry, 1 (11): 59–67, DOI: 10.1016/S0968-0896(02)00325-5.
Tong, S., Zhang, M., Wang, S., Yin, R., Yu, R., Wan, S., Jiang, T., and Zhang, L., 2016. Isothiouronium modification empowers pyrimidine-substituted curcumin analogs potent cytotoxicity and Golgi localization. European Journal of Medicinal Chemistry, (123): 849–857, DOI: 10.1016/j.ejmech.2016.07.071.
Yin, R., Jiang, L., Wan, S., and Jiang, T., 2015. Efficient syntheses of permethylated derivatives of neolamellarin A, a pyrrolic marine natural product. Journal of Ocean University of China, 2 (14): 329–334, DOI: 10.1007/s11802-015-2372-z.
Yin, R., Zhang, M., Hao, C., Wang, W., Qiu, P., Wan, S., Zhang, L., and Jiang, T., 2013. Different cytotoxicities and cellular localizations of novel quindoline derivatives with or without boronic acid modifications in cancer cells. Chemical Communications (Camb), 76 (49): 8516–8518, DOI: 10.1039/c3cc 45203d.
Zhang, P. Y., Wong, I. L., Yan, C. S., Zhang, X. Y., Jiang, T., Chow, L. M., and Wan, S. B., 2010. Design and syntheses of permethyl ningalin B analogues: Potent multidrug resistance (MDR) reversal agents of cancer cells. Journal of Medicinal Chemistry, 14 (53): 5108–5120, DOI: 10.1021/jm100035c.
Acknowledgements
This study is supported by the National Natural Science Foundation of China (Nos. 21171154 and 81672585), the Key Technology Fund of Shandong Province (No. 2016ZDJS07 A07), and the Taishan Scholar Fellowship of Shandong Province in China to L. Z.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Zhang, M., Yin, R., Zhang, Y. et al. Synthesis and Neuroprotective Activity of Neolamellarin A Analogues. J. Ocean Univ. China 17, 967–972 (2018). https://doi.org/10.1007/s11802-018-3530-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11802-018-3530-x