Abstract
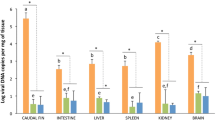
Lymphocystis disease virus (LCDV) infects target cells by attaching to a 27.8 kDa receptor (27.8R) protein in flounder Paralichthys olivaceus, and anti-27.8R monoclonal antibodies (MAbs) have been developed. However, the 27.8R existence in tissues of sea bass (Lateolabrax japonicus) and its role in LCDV infection have remained unclear. In this study, the results of western blotting demonstrated that the same 27.8R was shared by flounder and sea bass. LCDV-free sea bass individuals were intramuscularly injected with LCDV, and viral copies were detected in tissues from 3 h post infection and showed a time-dependent increase during 9 days infection. Distribution and synthesis of 27.8R in sea bass tissues were investigated by using anti-27.8R MAbs as probes. It was found that 27.8R was distributed in all the tested tissues. The levels of 27.8R protein were highest in gill and skin, then a bit lowly in stomach, head kidney and heart, followed by spleen, intestine, blood cells, gonad and liver, and least in kidney and brain in healthy sea bass. Upon LCDV infection, 27.8R synthesis was up-regulated in each tissue, and higher in the tissues with higher LCDV copies. The 27.8R and LCDV were detected in some peripheral blood leukocytes but not in red blood cells. These results suggested that 27.8R was widely distributed in sea bass tissues, and it served as a receptor and correlated with tissue tropism of LCDV infection. Furthermore, leukocytes had the potential of being a LCDV carrier and were responsible for a systemic infection of LCDV in sea bass.
Similar content being viewed by others
References
Alonso, M. C., Cano, I., Garcia-Rosado, E., Castro, D., Lamas, J., Barja, J. L., and Borrego, J. J., 2005. Isolation of lymphocystis disease virus from sole, Solea senegalensis Kaup, and blackspot sea bream, Pagellus bogaraveo (Brünnich). Journal of Fish Diseases, 28 (4): 221–228.
Bowser, P. R., Wooster, G. A., and Getchell, R. G., 1999. Transmission of walleye dermal sarcoma and lymphocystis via waterborne exposure. Journal of Aquatic Animal Health, 11 (2): 158–161.
Cano, I., Ferro, P., Alonso, M. C., Bergmann, S. M., Römer-Oberdö rfer, A., Garcia-Rosado, E., Castro, D., and Borrego, J. J., 2007. Development of molecular techniques for detection of lymphocystis disease virus in different marine fish species. Journal of Applied Microbiology, 102 (1): 32–40.
Cano, I., Valverde, E. J., Garcia-Rosado, E., Alonso, M. C., Lopez-Jimena, B., Ortiz-Delgado, J. B., Borrego J. J., Sarasquete, C., and Castro, D., 2013. Transmission of lymphocystis disease virus to cultured gilthead seabream, Sparus aurata L., larvae. Journal of Fish Diseases, 36 (6): 569–576.
Cheng, S. F., Zhan, W. B., Xing, J., and Sheng, X. Z., 2006. Development and characterization of monoclonal antibody to the lymphocystis disease virus of Japanese flounder Paralichthys olivaceus isolated from China. Journal of Virological Methods, 135 (2): 173–180.
Colorni, A., and Diamant, A., 1995. Splenic and cardiac lymphocystis in the red drum, Sciaenops ocellatus (L.). Journal of Fish Diseases, 18 (5): 467–471.
Dezfuli, B. S., Lui, A., Giari, L., Castaldelli, G., Mulero, V., and Noga, E. J., 2012. Infiltration and activation of acidophilic granulocytes in skin lesions of gilthead seabream, Sparus aurata, naturally infected with lymphocystis disease virus. Developmental and Comparative Immunology, 36 (1): 174–182.
Flint, M., and Tscherne, D. M., 2009. Cellular receptors and HCV entry. In: Hepatitis C: Methods and Protocols. Humana Press, New Jersey, 265–277.
Gibson-Kueh, S., Netto, P., Ngoh-Lim, G. H., Chang, S. F., and Ho, L. L., 2003. The pathology of systemic iridoviral disease in fish. Journal of Comparative Pathology, 129 (2): 111–119.
Harikrishnan, R., Balasundaram, C., and Heo, M. S., 2010. Effect of probiotics enriched diet on Paralichthys olivaceus infected with lymphocystis disease virus (LCDV). Fish & Shellfish Immunology, 29 (5): 868–874.
Iwakiri, S., Song, J. Y., Nakayama, K., Oh, M. J., Ishida, M., and Kitamura, S. I., 2014. Host responses of Japanese flounder Paralichthys olivaceus with lymphocystis cell formation. Fish & Shellfish Immunology, 38 (2): 406–411.
Iwamoto, R., Hasegawa, O., Lapatra, S., and Yoshimizu, M., 2002. Isolation and characterization of the Japanese flounder (Paralichthys olivaceus) lymphocystis disease virus. Journal of Aquatic Animal Health, 14: 114–123.
Kilkenny, C., Browne, W., Cuthill, I. C., Emerson, M., and Altman, D. G., 2010. Animal research: Reporting in vivo experiments: The ARRIVE guidelines. Journal of Gene Medicine, 12: 561–563.
Kvitt, H., Heinisch, G., and Diamant, A., 2008. Detection and phylogeny of Lymphocystivirus in sea bream Sparus aurata based on the DNA polymerase gene and major capsid protein sequences. Aquaculture, 275 (1): 58–63.
Okino, C. H., Santos, I. L., and Fernando, F. S., 2014. Inflammatory and cell-mediated immune responses in the respiratory tract of chickens to infection with avian infectious bronchitis virus. Viral Immunology, 27 (8): 383–391.
Papi, A., and Johnston, S. L., 1999. Rhinovirus infection induces expression of its own receptor intercellular adhesion molecule 1 (ICAM-1) via increased NF-κB-mediated transcription. Journal of Biological Chemistry, 274 (14): 9707–9720.
Qu, L. Y., Sun, X. Q., and Zhang, J. X., 2000. Electron ultramicroscope observation of lymphocystis disease of the left-eyed flounder Paralichthys olivaceus. Journal of Ocean University of China, 30 (2): 105–110.
Sheng, X. Z., Wang, M., Xing, J., and Zhan, W. B., 2012. Monoclonal antibodies against 27.8 kDa protein receptor efficiently block lymphocystis disease virus infection in flounder Paralichthys olivaceus gill cells. Diseases of Aquatic Organisms, 100 (1): 19–27.
Sheng, X. Z., Wu, R. H., Tang, X. Q., Xing, J., and Zhan, W. B., 2015. Tissue localization of lymphocystis disease virus (LCDV) receptor-27.8 kDa and its expression kinetics induced by the viral infection in turbot (Scophthalmus maximus). International Journal of Molecular Sciences, 16: 26506–26519.
Sheng, X. Z., Xu, X. L., and Zhan, W. B., 2013. Development and application of antibody microarray for lymphocystis disease virus detection in fish. Journal of Virological Methods, 189 (2): 243–249.
Sun, C., Hu, L. L., Liu, S. S., Hu, G. B., and Zhang, S. C., 2013. Antiviral activity of phosvitin from zebrafish Danio rerio. Developmental and Comparative Immunology, 40 (1): 28–34.
Valverde, E. J., Cano, I., Labella, A., Borrego, J. J., and Castro, D., 2016. Application of a new real-time polymerase chain reaction assay for surveillance studies of lymphocystis disease virus in farmed gilthead seabream. BMC Veterinary Research, 12 (1): 71.
Wang, M., Sheng, X. Z., Xing, J., Tang, X. Q., and Zhan, W. B., 2011. Identification of a 27.8 kDa protein from flounder gill cells involved in lymphocystis disease virus binding and infection. Diseases of Aquatic Organisms, 94 (1): 9–16.
Wang, S., Nie, Q., Zheng, L. Y., Hum, J., and Luo, E. J., 2010. In vivo kinetics and biodistribution of a Hantaan virus DNA vaccine after intramuscular injection in mice. Virologica Sinica, 25 (3): 177–182.
Wenzlow, N., Plattet, P., Wittek, R., Zurbriggen, A., and Grone, A., 2007. Immunohistochemical demonstration of the putative canine distemper virus receptor CD150 in dogs with and without distemper. Vet Pathology, 44 (6): 943–948.
Wolf, K., 1988. Fish Viruses and Fish Viral Diseases. Comstock Publishing Associates, Cornell University Press, New York, 821pp.
Wu, R. H., Tang, X. Q., Sheng, X. Z., and Zhan, W. B., 2015a. Relationship between expression of cellular receptor-27.8 kDa and lymphocystis disease virus (LCDV) infection. PLoS ONE, 10: e0127940.
Wu, R. H., Tang, X. Q., Sheng, X. Z., and Zhan, W. B., 2015b. Tissue distribution of the 27.8 kDa receptor and its dynamic expression in response to lymphocystis disease virus infection in flounder (Paralichthys olivaceus). Journal of Comparative Pathology, 153: 324–332
Yang, C. G., Wang, X. L., Wang, L., Zhang, B., and Chen, S. L., 2011. A new Akirin1 gene in turbot (Scophthalmus maximus): Molecular cloning, characterization and expression analysis in response to bacterial and viral immunological challenge. Fish & Shellfish Immunology, 30: 1031–1041.
Zhan, W. B., Li, Y. Q., Sheng, X. Z., Xing, J., and Tang, X. Q., 2010. Detection of lymphocystis disease virus in Japanese flounder Paralichthys olivaceus and other marine teleosts from northern China. Chinese Journal of Oceanology and Limnology, 28: 1213–1220.
Zhan, W. B., Wang, Y. H., Fryer, J. L., Okubo, K., and Fukuda, H., 1999. Production of monoclonal antibodies (MAbs) against white spot syndrome virus (WSSV). Journal of Aquatic Animal Health, 11: 17–22.
Zhang, Q. Y., and Gui, J. F., 2015. Virus genomes and virus-host interactions in aquaculture animals. Science China Life Sciences, 58 (2): 156–169.
Zhang, Q. Y., Xiao, F., Xie, J., Li, Z. Q., and Gui, J. F., 2004. Complete genome sequence of lymphocystis disease virus isolated from China. Journal of Virology, 78: 6982–6994.
Zheng, F. R., Sun, X. Q., Xing, M. Q., and Liu, H., 2010. Immune response of DNA vaccine against lymphocystis disease virus and expression analysis of immune-related genes after vaccination. Aquaculture Research, 41 (10): 1444–1451.
Zhong, R. J., Tang, X. Q., Zhan, W. B., Xing, J., and Sheng, X. Z., 2013. Expression kinetics of β-integrin in Chinese shrimp (Fenneropenaeus chinensis) hemocytes following infection with white spot syndrome virus. Fish & Shellfish Immunology, 35: 539–545.
Acknowledgements
This study is supported by the National Natural Science Foundation of China (Nos. 31472295 and 31672685), Science and Technology Development Project of Shandong Province (No. 2014GNC111015) and Taishan Scholar Program of Shandong Province.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wu, R., Sheng, X., Tang, X. et al. Localization and dynamic expression of a 27.8 kDa receptor protein for lymphocystis disease virus infection in sea bass (Lateolabrax japonicus) tissues. J. Ocean Univ. China 16, 889–896 (2017). https://doi.org/10.1007/s11802-017-3280-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11802-017-3280-1