Abstract
A high level of lipoprotein(a) (Lp(a)) is recognized as an independent and additional cardiovascular risk factor contributing to the risk of early onset and progressive course of cardiovascular disease (CVD). All lipid lowering medications in use mainly lower low density lipoprotein-cholesterol (LDL-c) with no or limited effect on levels of Lp(a). Niacin, the only component lowering Lp(a), is firstly often poorly tolerated and secondly not available anymore in many countries. A level of <50 mg/dl was recommended recently as the cut off level for clinical use and decision making. Since lipoprotein apheresis (LA) lowers not only LDL-c but also Lp(a) significantly, its use is recommended in some countries in very high-risk patients with early or progressive CVD. Retrospective analyses show that regular LA improves the course of CVD. This is supported by a recent prospective observational trial and data of the German Lipoprotein Apheresis Registry. Despite many treatment options, all too often it is not possible to reduce LDL-c levels to target and to reduce Lp(a) levels sustainably at all. Therefore, new drug therapies are awaited. Some of the lipid modifying drugs in development lower Lp(a) to some extent in addition to LDL-c; the only specific approach is the apoprotein(a) antisense oligonucleotide. Currently LA is the standard of care as a last resort treatment in high-risk patients with elevated Lp(a) and severe CVD despite optimal control of all other cardiovascular risk factors.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Lipoprotein(a) (Lp(a)) in man was first described in 1963 by Berg, who stated that the level of Lp(a) is mainly inherited and that high levels of Lp(a) are associated with premature atherosclerosis [1]. This has been confirmed thereafter by observational data and supported in recent years by genetic data establishing Lp(a) as a causal factor for the development of atherosclerosis [2–8]. The European Guidelines on vascular disease prevention in clinical practice [9] mention high levels of Lp(a) as being associated with an increased risk of CVD and suggest to use levels of Lp(a) for risk stratification in individuals at moderate risk or with a positive family history of early CVD. The consensus paper of the European Atherosclerosis Society (EAS) [10] offers guidance. Lp(a) should be measured e. g. in all individuals at intermediate or high risk of CVD, in case of premature CVD, familial hypercholesterolaemia, premature CVD or high Lp(a) in the family, progressive CVD despite statin therapy.
Lp(a) consists of a low density lipoprotein (LDL) particle and an additional protein called apoprotein(a) (apo(a)), linked to apoprotein B (apoB) 100 of the LDL particle via one disulfide bond. Mostly the level depends on the size of apo(a) [11] and both are negatively correlated. The different laboratory methods are not comparable and results cannot be converted. If comparing data the used methods have to be taken into account. The consensus statement of the EAS recommended the use of an isoform insensitive assay and suggested a level of <50 mg/dl as desirable [10]. Since risk increases with increasing levels of Lp(a) and interventional data are missing, there is no established threshold.
Established therapies
Drugs
Some data show either a decrease or an increase of Lp(a) by statins [12], but mostly Lp(a) is not affected by statins [10, 13, 14] nor by ezetimibe [15]. Nicotinic acid (niacin) reduces Lp(a) besides positive effects on LDL-c, high density lipoprotein-cholesterol (HDL-c), and triglycerides [16]; high doses (2–4 g) reduce Lp(a) significantly [17]. Whether this holds true for individuals with high levels of Lp(a) has never been shown. A meta-analysis of the beneficial effects of nicotinic acid on cardiovascular events [18] did not discriminate whether the lowered levels of Lp(a) might have contributed to the positive results or not. It has to be mentioned that these data are mainly from the pre-statin era and would have to be confirmed in cohorts treated in line with current options and guidelines. In the EAS consensus paper niacin is recommended to reduce high levels of Lp(a) [10]. Since 2013 niacin is not available in Europe. In summary, no established drug treatment option to reduce Lp(a) is available at the moment.
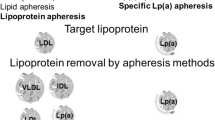
Lipoprotein apheresis
Lipoprotein apheresis (LA) is in clinical use for over 30 years [19] and reduces apoB100 containing lipoproteins (namely LDL-c and Lp(a)). A single treatment reduces both by about 60–70%. The following increase is rapid [20]. For this reason, LA has to be repeated regularly and is done every week in most or every two weeks in some countries. Guidelines of several countries recommend LA in very high risk patients as a last resort therapy to lower LDL-c in addition to maximal (tolerated) lipid lowering medication. Few countries also consider high levels of Lp(a) as an indication for LA in very high risk patients [21–24].
There are no randomised prospective trials. LA has beneficial effects regarding endothelial function and myocardial perfusion in patients with high levels of Lp(a) [25]. Retrospective evaluations of clinical data and analyses of the German Lipoprotein Apheresis Registry (GLAR) show that cardiovascular events were reduced significantly after establishing regular LA [26–28]. One retrospective evaluation indicates that patients with elevated Lp(a) irrespective of the LDL-c level have a greater benefit from LA than patients with low Lp(a) and high levels of LDL-c [29]. One prospective open-label trial used atorvastatin plus the a selective Lp(a) lowering apheresis system (treatment group) and atorvastatin alone (control group). After 18 months a small but significant regression of coronary atherosclerosis was documented by angiography in the LA group [30]. The Pro(a)-LiFe-study, a non-randomised prospective observational multicentre study in high-risk patients, showed a reduction of major adverse cardiac events and of major adverse non-cardiac events after two [31] as well as after five years [32] of apheresis therapy.
Lipoprotein apheresis is well tolerated and safe in clinical use [24, 33] even for 2–3 decades of treatment. Several obstacles are opposed to a wide clinical use: it is time consuming, expensive, only offered in specialised centres, and mostly not covered by insurance companies.
Emerging therapies
In recent years several new lipid modifying approaches mostly addressing LDL-c were investigated. Inhibitors of the cholesterol ester transfer protein (CETP) increasing HDL-c and reducing LDL-c are not approved yet. In a phase-2 trial Anazetrapib additionally lowered Lp(a) by 50% [34]. In the REALIZE trial Anazetrapib showed a substantial reduction of Lp(a) by 31.8% in patients with heterozygous familial hypercholesterolaemia (FH) [35]. TA-8995, a newer agent, lowered Lp(a) in patients with dyslipidaemia by 36.9% (5 mg) and 33.4% (10 mg) at week 12 in a phase 2 trial [36]. The REVEAL trial using Anacetrapib to be published in 2017 will show whether this lipid modifying approach is beneficial in patients with established vascular disease [37].
The apoB antisense oligonucleotide (ASO) Mipomersen is approved in the US but not in Europe [38, 39]. Besides the reduction of LDL-c via less hepatic production of very low density lipoprotein (VLDL) Lp(a) is reduced by a still unclear mechanism. In a randomized trial Mipomersen [40] reduced initially elevated levels of Lp(a) significant by 31.1%. In patients with heterozygous FH and coronary heart disease Mipomersen was added to lipid lowering therapy and reduced Lp(a) by 21.1% [41]. Thus, Mipomersen might reduce the necessity for lipoprotein apheresis in high-risk patients [42].
Lomitapide, an inhibitor of the microsomal triglyceride transfer protein (MTP), lowers LDL-c by reducing the assembly of VLDL in the liver as well of chylomicrons in the intestine. This is the only approach independent of the functionality of LDL-receptors. For this reason, the MTP-inhibitor Lomitapide significantly lowers LDL-c in homozygous FH (hoFH). Efficacy and safety in patients with hoFH were assessed in a single-arm, open-label, phase-3 study. Lp(a) was reduced by 19% at week 56 but at week 78 this was no longer seen [43]. Lomitapide is approved for hoFH.
PCSK9-inhibitors
Like the LDL-receptor the proprotein convertase subtilisin/kexin type 9 (PCSK9) is produced in the liver cells and released into the circulation. If PCSK9 is attached to the complex of LDL-receptor and LDL-c the receptor is degraded after internalisation and thus cannot be recycled for further uptake of LDL-c. Loss of function mutations come along with lower levels of LDL-c lifelong and are linked to a lower rate of cardiovascular events [44]. Two PCSK9-inhibitors (fully humanized monoclonal antibodies) to mimic these beneficial “natural” effects by binding PCSK9 and thus hampering the intracellular lysis of the LDL-receptor are approved. Overall, safety and tolerability profiles are very promising in both of the huge trial programmes. Long-term results show a sustained significant reduction of LDL-c [45, 46]. The LDL-c lowering effect is dependent on the functionality of the LDL-receptors and can vary widely. Mean LDL-c reduction is about 50–60% and in some trials a lowering of Lp(a) [45–48] was seen as well (Evolocumab 25.5% [46], Alirocumab 30.2% [45]).
A pooled analysis [48] of three phase-2 Alirocumab trials specifically analysed the effect on Lp(a). Baseline levels between 2 and 181 mg/dl were parted in two groups (≤ and ≥50 mg/dl). In absolute values Lp(a) was reduced by 3.5 mg/dl and 27 mg/dl (mean), respectively, or relatively by 36% and 27% (median), respectively. In patients with LDL-receptor negative hoFH evolocumab was, as expected, ineffective in reducing LDL-c but all the same Lp(a) was lowered by 20% [49]. The upregulation of other receptors, e. g. the VLDL-receptor that mediates the uptake of Lp(a) into macrophages, might be an explanation for this finding. Some data show an increased catabolic fraction rate, others a reduced synthesis [50]. The development of bococizumab was terminated in November 2016 due to an unexpected decline of effectivity over time, a higher level of immunogenicity, and more frequent injection site reactions [51].
The results of the endpoint trials are expected in early 2017 (Evolocumab [52]) and in 2018 (Alirocumab [53]). Whether lowering Lp(a) by PCSK9-inhibitors contributes to the expected beneficial cardiovascular effects will have to be addressed in specifically designed trials.
Apo(a) antisense oligonucleotide
The ASO molecule IONIS-APO(a)-Rx specifically addresses the mRNA of apo(a). After a positive trial in transgenic mice [54] a phase-1 trial in healthy volunteers with Lp(a) levels ≥25 nmol/l was conducted. Various doses of the ASO lowered Lp(a) significantly (no relevant reduction of other lipoproteins). The highest dose of 300 mg reduced Lp(a) by 77.8%. This effect was sustained and Lp(a) was still lowered 84 days after the last dose [55]. Data of a phase-2 trial are awaited. If effectivity and safety are shown this ASO would for the first time allow trials to address the question if the isolated lowering of high levels of Lp(a) results in lesser CVD.
Conclusion
The new drug developments will enable us to reduce LDL-c as significantly as never before with more high-risk patients reaching their treatment goals. The results of the endpoint trials will show to what extent this contributes to the expected reduction of cardiovascular events and deaths. The question if the concomitant lowering effect of Lp(a) is beneficial in addition has never been addressed so far. This will have to be evaluated in trials specifically addressing patients with high levels of Lp(a) and CVD. It is far more reasonable to address this question with the apo(a) ASO. So far, there are no data for any established or newly developed agent proving that the Lp(a) lowering effect is beneficial in high-risk cardiovascular patients.
All available data regarding lipoprotein apheresis, though not from RCTs, at least strongly support its beneficial effect of reducing cardiovascular events. In patients with controlled cardiovascular risk factors, LDL-c at goal, progressive cardiovascular disease, and markedly elevated levels of Lp(a) lipoprotein apheresis remains the only available and optimal care. As of today, it should be considered in high-risk patients wherever it is available.
References
Berg K (1963) A new serum type system in man – the Lp system. Acta Pathol Microbiol Scand 59:369–382
Clarke R, Peden JF, Hopewell JC, Kyriakou T, Goel A, Heath SC, Parish S, Barlera S, Franzosi MG, Rust S, Bennett D, Silveira A, Malarstig A, Green FR, Lathrop M, Gigante B, Leander K, de Faire U, Seedorf U, Hamsten A, Collins R, Watkins H, Farrall M, Consortium P (2009) Genetic variants associated with Lp(a) lipoprotein level and coronary disease. N Engl J Med 361(26):2518–2528. doi:10.1056/NEJMoa0902604
Kamstrup PR, Tybjaerg-Hansen A, Nordestgaard BG (2014) Elevated lipoprotein(a) and risk of aortic valve stenosis in the general population. J Am Coll Cardiol 63(5):470–477. doi:10.1016/j.jacc.2013.09.038
Kamstrup PR, Tybjaerg-Hansen A, Steffensen R, Nordestgaard BG (2009) Genetically elevated lipoprotein(a) and increased risk of myocardial infarction. JAMA 301(22):2331–2339. doi:10.1001/jama.2009.801
Kronenberg F, Kronenberg MF, Kiechl S, Trenkwalder E, Santer P, Oberhollenzer F, Egger G, Utermann G, Willeit J (1999) Role of lipoprotein(a) and apolipoprotein(a) phenotype in atherogenesis: prospective results from the Bruneck study. Circulation 100(11):1154–1160
Kronenberg F, Neyer U, Lhotta K, Trenkwalder E, Auinger M, Pribasnig A, Meisl T, Konig P, Dieplinger H (1999) The low molecular weight apo(a) phenotype is an independent predictor for coronary artery disease in hemodialysis patients: a prospective follow-up. J Am Soc Nephrol 10(5):1027–1036
Sandholzer C, Saha N, Kark JD, Rees A, Jaross W, Dieplinger H, Hoppichler F, Boerwinkle E, Utermann G (1992) Apo(a) isoforms predict risk for coronary heart disease. A study in six populations. Arterioscler Thromb 12(10):1214–1226
Kraft HG, Lingenhel A, Kochl S, Hoppichler F, Kronenberg F, Abe A, Muhlberger V, Schonitzer D, Utermann G (1996) Apolipoprotein(a) kringle IV repeat number predicts risk for coronary heart disease. Arterioscler Thromb Vasc Biol 16(6):713–719
Authors/Task Force M, Piepoli MF, Hoes AW, Agewall S, Albus C, Brotons C, Catapano AL, Cooney MT, Corra U, Cosyns B, Deaton C, Graham I, Hall MS, Hobbs FD, Lochen ML, Lollgen H, Marques-Vidal P, Perk J, Prescott E, Redon J, Richter DJ, Sattar N, Smulders Y, Tiberi M, van der Worp HB, van Dis I, Verschuren WM, Additional Contributor, Simone B, Document R, De Backer G, Roffi M, Aboyans V, Bachl N, Bueno H, Carerj S, Cho L, De Sutter J, Egidi G, Fisher M, Fitzsimons D, Franco OH, Guenoun M, Jennings C, Jug B, Kirchhof P, Kotseva K, Lip GY, Mach F, Mancia G, Bermudo FM, Mezzani A, Niessner A, Ponikowski B, Rauch B, Ryden L, Stauder A, Turc G, Wiklund O, Windecker S, Zamorano JL (2016) 2016 European Guidelines on cardiovascular disease prevention in clinical practice: The Sixth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of 10 societies and by invited experts): Developed with the special contribution of the European Association for Cardiovascular Prevention & Rehabilitation (EACPR). Eur J Prev Cardiol 23(11):NP1-NP96. doi:10.1177/2047487316653709
Nordestgaard BG, Chapman MJ, Ray K, Boren J, Andreotti F, Watts GF, Ginsberg H, Amarenco P, Catapano A, Descamps OS, Fisher E, Kovanen PT, Kuivenhoven JA, Lesnik P, Masana L, Reiner Z, Taskinen MR, Tokgozoglu L, Tybjaerg-Hansen A (2010) Lipoprotein(a) as a cardiovascular risk factor: current status. Eur Heart J 31(23):2844–2853. doi:10.1093/eurheartj/ehq386
Utermann G (1989) The mysteries of lipoprotein(a). Science 246(4932):904–910
Khera AV, Everett BM, Caulfield MP, Hantash FM, Wohlgemuth J, Ridker PM, Mora S (2014) Lipoprotein(a) concentrations, rosuvastatin therapy, and residual vascular risk: an analysis from the JUPITER trial (justification for the use of statins in prevention: an intervention trial evaluating rosuvastatin). Circulation 129(6):635–642. doi:10.1161/CIRCULATIONAHA.113.004406
Tziomalos K, Athyros VG, Wierzbicki AS, Mikhailidis DP (2009) Lipoprotein a: where are we now? Curr Opin Cardiol 24(4):351–357. doi:10.1097/HCO.0b013e32832ac21a
Kei A, Liberopoulos E, Tellis K, Rizzo M, Elisaf M, Tselepis A (2013) Effect of hypolipidemic treatment on emerging risk factors in mixed dyslipidemia: a randomized pilot trial. Eur J Clin Invest 43(7):698–707. doi:10.1111/eci.12095
Moutzouri E, Liberopoulos EN, Tellis CC, Milionis HJ, Tselepis AD, Elisaf MS (2013) Comparison of the effect of simvastatin versus simvastatin/ezetimibe versus rosuvastatin on markers of inflammation and oxidative stress in subjects with hypercholesterolemia. Atherosclerosis 231(1):8–14. doi:10.1016/j.atherosclerosis.2013.08.013
Carlson LA, Hamsten A, Asplund A (1989) Pronounced lowering of serum levels of lipoprotein Lp(a) in hyperlipidaemic subjects treated with nicotinic acid. J Intern Med 226(4):271–276
Goldberg A, Alagona P Jr., Capuzzi DM, Guyton J, Morgan JM, Rodgers J, Sachson R, Samuel P (2000) Multiple-dose efficacy and safety of an extended-release form of niacin in the management of hyperlipidemia. Am J Cardiol 85(9):1100–1105. doi:S0002-9149(00)00703-7
Bruckert E, Labreuche J, Amarenco P (2010) Meta-analysis of the effect of nicotinic acid alone or in combination on cardiovascular events and atherosclerosis. Atherosclerosis 210(2):353–361. doi:10.1016/j.atherosclerosis.2009.12.023
Abel J (1914) Plasma removal with return of corpuscles. J Pharmacol Exp Ther 5(6):625–641
Thompson GR (2010) Lipoprotein apheresis. Curr Opin Lipidol 21(6):487–491. doi:10.1097/MOL.0b013e32833e13
Bundesausschuss G (2010) Richtlinie des Gemeinsamen Bundesausschusses zu Untersuchungs- und Behandlungsmethoden der vertragsärztlichen Versorgung. Bundesanz 109:2561
Derfler K, Steiner S, Sinzinger H (2015) Lipoprotein-apheresis: Austrian consensus on indication and performance of treatment. Wien Klin Wochenschr 127(15–16):655–663. doi:10.1007/s00508-015-0833-4
Stefanutti C (2010) The 2009 2nd Italian Consensus Conference on LDL-apheresis. Nutr Metab Cardiovasc Dis 20(10):761–762. doi:10.1016/j.numecd.2010.04.007
Thompson GR (2008) Recommendations for the use of LDL apheresis. Atherosclerosis 198(2):247–255. doi:10.1016/j.atherosclerosis.2008.02.009
Bohl S, Kassner U, Eckardt R, Utz W, Mueller-Nordhorn J, Busjahn A, Thomas HP, Abdel-Aty H, Klingel R, Marcovina S, Dietz R, Steinhagen-Thiessen E, Schulz-Menger J, Vogt A (2009) Single lipoprotein apheresis session improves cardiac microvascular function in patients with elevated lipoprotein(a): detection by stress/rest perfusion magnetic resonance imaging. Ther Apher Dial 13(2):129–137. doi:10.1111/j.1744-9987.2009.00667.x
Jaeger BR, Richter Y, Nagel D, Heigl F, Vogt A, Roeseler E, Parhofer K, Ramlow W, Koch M, Utermann G, Labarrere CA, Seidel D, Group of (2009) Longitudinal cohort study on the effectiveness of lipid apheresis treatment to reduce high lipoprotein(a) levels and prevent major adverse coronary events. Nat Clin Pract Cardiovasc Med 6(3):229–239. doi:10.1038/ncpcardio1456
Rosada A, Kassner U, Vogt A, Willhauck M, Parhofer K, Steinhagen-Thiessen E (2014) Does regular lipid apheresis in patients with isolated elevated lipoprotein(a) levels reduce the incidence of cardiovascular events? Artif Organs 38(2):135–141. doi:10.1111/aor.12135
Schettler VJ, Neumann CL, Peter C, Zimmermann T, Julius U, Roeseler E, Heigl F, Ramlow W, Blume H, Scientific Board of GftGAWG (2015) First data from the German Lipoprotein Apheresis Registry (GLAR). Atheroscler Suppl 18:41–44. doi:10.1016/j.atherosclerosissup.2015.02.006
von Dryander M, Fischer S, Passauer J, Muller G, Bornstein SR, Julius U (2013) Differences in the atherogenic risk of patients treated by lipoprotein apheresis according to their lipid pattern. Atheroscler Suppl 14(1):39–44. doi:10.1016/j.atherosclerosissup.2012.10.005
Safarova MS, Ezhov MV, Afanasieva OI, Matchin YG, Atanesyan RV, Adamova IY, Utkina EA, Konovalov GA, Pokrovsky SN (2013) Effect of specific lipoprotein(a) apheresis on coronary atherosclerosis regression assessed by quantitative coronary angiography. Atheroscler Suppl 14(1):93–99. doi:10.1016/j.atherosclerosissup.2012.10.015
Leebmann J, Roeseler E, Julius U, Heigl F, Spitthoever R, Heutling D, Breitenberger P, Maerz W, Lehmacher W, Heibges A, Klingel R, ProLiFe Study G (2013) Lipoprotein apheresis in patients with maximally tolerated lipid-lowering therapy, lipoprotein(a)-hyperlipoproteinemia, and progressive cardiovascular disease: prospective observational multicenter study. Circulation 128(24):2567–2576. doi:10.1161/CIRCULATIONAHA.113.002432
Roeseler E, Julius U, Heigl F, Spitthoever R, Heutling D, Breitenberger P, Leebmann J, Lehmacher W, Kamstrup PR, Nordestgaard BG, Maerz W, Noureen A, Schmidt K, Kronenberg F, Heibges A, Klingel R, ProLiFe-Study G (2016) Lipoprotein Apheresis for Lipoprotein(a)-Associated Cardiovascular Disease: Prospective 5 Years of Follow-Up and Apolipoprotein(a) Characterization. Arterioscler Thromb Vasc Biol 36(9):2019–2027. doi:10.1161/ATVBAHA.116.307983
Thompson GR, Lowenthal R, Myant NB (1975) Plasma exchange in the management of homozygous familial hypercholesterolaemia. Lancet 1(7918):1208–1211. doi:10.1016/s0140-6736(75)92193-5
Teramoto T, Shirakawa M, Kikuchi M, Nakagomi M, Tamura S, Surks HK, McCrary Sisk C, Numaguchi H (2013) Efficacy and safety of the cholesteryl ester transfer protein inhibitor anacetrapib in Japanese patients with dyslipidemia. Atherosclerosis 230(1):52–60. doi:10.1016/j.atherosclerosis.2013.05.012
Kastelein JJ, Besseling J, Shah S, Bergeron J, Langslet G, Hovingh GK, Al-Saady N, Koeijvoets M, Hunter J, Johnson-Levonas AO, Fable J, Sapre A, Mitchel Y (2015) Anacetrapib as lipid-modifying therapy in patients with heterozygous familial hypercholesterolaemia (REALIZE): a randomised, double-blind, placebo-controlled, phase 3 study. Lancet 385(9983):2153–2161. doi:10.1016/S0140-6736(14)62115-2
Hovingh GK, Kastelein JJ, van Deventer SJ, Round P, Ford J, Saleheen D, Rader DJ, Brewer HB, Barter PJ (2015) Cholesterol ester transfer protein inhibition by TA-8995 in patients with mild dyslipidaemia (TULIP): a randomised, double-blind, placebo-controlled phase 2 trial. Lancet 386(9992):452–460. doi:10.1016/S0140-6736(15)60158-1
Randomized EValuation of the Effects of Anacetrapib Through Lipid-modification (REVEAL). https://clinicaltrials.gov/ct2/show/NCT01252953
Rader DJ, Kastelein JJ (2014) Lomitapide and mipomersen: two first-in-class drugs for reducing low-density lipoprotein cholesterol in patients with homozygous familial hypercholesterolemia. Circulation 129(9):1022–1032. doi:10.1161/CIRCULATIONAHA.113.001292
Parhofer KG (2012) Mipomersen: evidence-based review of its potential in the treatment of homozygous and severe heterozygous familial hypercholesterolemia. Core Evid 7:29–38. doi:10.2147/CE.S25239ce-7-029
Raal FJ, Santos RD, Blom DJ, Marais AD, Charng MJ, Cromwell WC, Lachmann RH, Gaudet D, Tan JL, Chasan-Taber S, Tribble DL, Flaim JD, Crooke ST (2010) Mipomersen, an apolipoprotein B synthesis inhibitor, for lowering of LDL cholesterol concentrations in patients with homozygous familial hypercholesterolaemia: a randomised, double-blind, placebo-controlled trial. Lancet 375(9719):998–1006. doi:10.1016/S0140-6736(10)60284-X
Stein EA, Dufour R, Gagne C, Gaudet D, East C, Donovan JM, Chin W, Tribble DL, McGowan M (2012) Apolipoprotein B synthesis inhibition with mipomersen in heterozygous familial hypercholesterolemia: results of a randomized, double-blind, placebo-controlled trial to assess efficacy and safety as add-on therapy in patients with coronary artery disease. Circulation 126(19):2283–2292. doi:10.1161/CIRCULATIONAHA.112.104125
Vogt A, Parhofer KG (2013) The potential of mipomersen, an ApoB synthesis inhibitor, to reduce necessity for LDL-apheresis in patients with heterozygous familial hypercholesterolemia and coronary artery disease. Expert Opin Pharmacother 14(6):691–697. doi:10.1517/14656566.2013.779253
Cuchel M, Meagher EA, du Toit Theron H, Blom DJ, Marais AD, Hegele RA, Averna MR, Sirtori CR, Shah PK, Gaudet D, Stefanutti C, Vigna GB, Du Plessis AM, Propert KJ, Sasiela WJ, Bloedon LT, Rader DJ, Phase 3 Ho FHLSi (2013) Efficacy and safety of a microsomal triglyceride transfer protein inhibitor in patients with homozygous familial hypercholesterolaemia: a single-arm, open-label, phase 3 study. Lancet 381(9860):40–46. doi:10.1016/S0140-6736(12)61731-0
Cohen JC, Boerwinkle E, Mosley TH Jr., Hobbs HH (2006) Sequence variations in PCSK9, low LDL, and protection against coronary heart disease. N Engl J Med 354(12):1264–1272. doi:10.1056/NEJMoa054013
Robinson JG, Farnier M, Krempf M, Bergeron J, Luc G, Averna M, Stroes ES, Langslet G, Raal FJ, Shahawy ME, Koren MJ, Lepor NE, Lorenzato C, Pordy R, Chaudhari U, Kastelein JJ, Investigators OLT (2015) Efficacy and safety of alirocumab in reducing lipids and cardiovascular events. N Engl J Med. doi:10.1056/NEJMoa1501031
Sabatine MS, Giugliano RP, Wiviott SD, Raal FJ, Blom DJ, Robinson J, Ballantyne CM, Somaratne R, Legg J, Wasserman SM, Scott R, Koren MJ, Stein EA, Open-Label Study of Long-Term Evaluation against LDLCI (2015) Efficacy and safety of evolocumab in reducing lipids and cardiovascular events. N Engl J Med. doi:10.1056/NEJMoa1500858
Raal FJ, Giugliano RP, Sabatine MS, Koren MJ, Langslet G, Bays H, Blom D, Eriksson M, Dent R, Wasserman SM, Huang F, Xue A, Albizem M, Scott R, Stein EA (2014) Reduction in lipoprotein(a) with PCSK9 monoclonal antibody evolocumab (AMG 145): a pooled analysis of more than 1,300 patients in 4 phase II trials. J Am Coll Cardiol 63(13):1278–1288. doi:10.1016/j.jacc.2014.01.006
Gaudet D, Kereiakes DJ, McKenney JM, Roth EM, Hanotin C, Gipe D, Du Y, Ferrand AC, Ginsberg HN, Stein EA (2014) Effect of alirocumab, a monoclonal proprotein convertase subtilisin/kexin 9 antibody, on lipoprotein(a) concentrations (a pooled analysis of 150 mg every two weeks dosing from phase 2 trials). Am J Cardiol 114(5):711–715. doi:10.1016/j.amjcard.2014.05.060
Raal FJ, Honarpour N, Blom DJ, Hovingh GK, Xu F, Scott R, Wasserman SM, Stein EA, Investigators T (2015) Inhibition of PCSK9 with evolocumab in homozygous familial hypercholesterolaemia (TESLA Part B): a randomised, double-blind, placebo-controlled trial. Lancet 385(9965):341–350. doi:10.1016/S0140-6736(14)61374-X
Desai NR, Kohli P, Giugliano RP, O’Donoghue ML, Somaratne R, Zhou J, Hoffman EB, Huang F, Rogers WJ, Wasserman SM, Scott R, Sabatine MS (2013) AMG145, a monoclonal antibody against proprotein convertase subtilisin kexin type 9, significantly reduces lipoprotein(a) in hypercholesterolemic patients receiving statin therapy: an analysis from the LDL-C assessment with proprotein convertase subtilisin kexin type 9 monoclonal antibody inhibition combined with statin therapy (LAPLACE)-thrombolysis in myocardial infarction (TIMI) 57 trial. Circulation 128(9):962–969. doi:10.1161/CIRCULATIONAHA.113.001969
Pfizer (2016) Pfizer discontinues global development of bococizumab, its investigational PCSK9 inhibitor. Pfizer, New York
Sabatine MS, Giugliano RP, Keech A, Honarpour N, Wang H, Liu T, Wasserman SM, Scott R, Sever PS, Pedersen TR (2016) Rationale and design of the further cardiovascular outcomes research with PCSK9 inhibition in subjects with elevated risk trial. Am Heart J 173:94–101. doi:10.1016/j.ahj.2015.11.015
Schwartz GG, Bessac L, Berdan LG, Bhatt DL, Bittner V, Diaz R, Goodman SG, Hanotin C, Harrington RA, Jukema JW, Mahaffey KW, Moryusef A, Pordy R, Roe MT, Rorick T, Sasiela WJ, Shirodaria C, Szarek M, Tamby JF, Tricoci P, White H, Zeiher A, Steg PG (2014) Effect of alirocumab, a monoclonal antibody to PCSK9, on long-term cardiovascular outcomes following acute coronary syndromes: rationale and design of the ODYSSEY outcomes trial. Am Heart J 168(5):682–689. doi:10.1016/j.ahj.2014.07.028
Merki E, Graham M, Taleb A, Leibundgut G, Yang X, Miller ER, Fu W, Mullick AE, Lee R, Willeit P, Crooke RM, Witztum JL, Tsimikas S (2011) Antisense oligonucleotide lowers plasma levels of apolipoprotein (a) and lipoprotein (a) in transgenic mice. J Am Coll Cardiol 57(15):1611–1621. doi:10.1016/j.jacc.2010.10.052
Tsimikas S, Viney NJ, Hughes SG, Singleton W, Graham MJ, Baker BF, Burkey JL, Yang Q, Marcovina SM, Geary RS, Crooke RM, Witztum JL (2015) Antisense therapy targeting apolipoprotein(a): a randomised, double-blind, placebo-controlled phase 1 study. Lancet 386(10002):1472–1483. doi:10.1016/S0140-6736(15)61252-1
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
A. Vogt has received speakers’ honoraria for presentations and advisory board activities by Merck Sharp & Dohme, Genzyme, a Sanofi company, Kaneka, Fresenius, BBraun, Amgen, Regeneron, Sanofi. A. Vogt has received research support by Merck Sharp & Dohme.
Additional information
This article is part of the special issue “Lp(a) – the underestimated cardiovascular risk factor”
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Vogt, A. Hyperlipoproteinaemia(a) – apheresis and emerging therapies. Clin Res Cardiol Suppl 12 (Suppl 1), 12–17 (2017). https://doi.org/10.1007/s11789-017-0083-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11789-017-0083-2