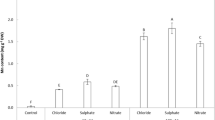
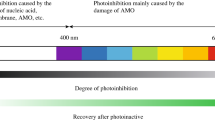
Abstract
Manganese oxides are widely distributed in soils and sediments, affecting the migration and transformation of heavy metals and organic pollutants. The microbial conversion of soluble Mn(II) into insoluble Mn(III/IV) oxides is considered to be the initial source of manganese oxides in the environment; however, whether this process is related to a physiological role remains unclear. Here, we explored the microbial manganese oxidation process under visible light by using coastal surface seawater microorganisms. Visible light greatly promotes the oxidation rate of Mn(II), and the average rate reaches 64 µmol/(L·d). The generated manganese oxides were then conducive to Mn(II) oxidation, thus the rapid manganese oxidation was the result of the combined action of biotic and abiotic, and biological function accounts for 88 % ± 4 %. Extracellular superoxide produced by microorganisms induced by visible light is the decisive factor for the rapid manganese oxidation in our study. But the production of these superoxides does not require the presence of Mn(II) ions, the Mn(II) oxidation process was more like an unintentional side reaction, which did not affect the growth of microorganisms. More than 70 % of heterotrophic microorganisms in nature are capable of producing superoxide, based on the oxidizing properties of free radicals, all these bacteria can participate in the geochemical cycle of manganese. What’s more, the superoxide oxidation pathway might be a significant natural source of manganese oxide.

Similar content being viewed by others
References
Andeer P F, Learman D R, McIlvin M, Dunn J A, Hansel C M (2015). Extracellular haem peroxidases mediate Mn(II) oxidation in a marine Roseobacter bacterium via superoxide production. Environmental Microbiology, 17(10): 3925–3936
Anderson C R, Johnson H A, Caputo N, Davis R E, Torpey J W, Tebo B M (2009). Mn(II) oxidation is catalyzed by heme peroxidases in “Aurantimsnas mangansxydans” strain SI85-9A1 and Erythrobacter sp. strain SD-21. Applied and Environmental Microbiology, 75(12): 4130–4138
Bender M L, Klinkhammer G P, Spencer D W (1977). Manganese in seawater and the marine manganese balance. Deep-Sea Research, 24(9): 799–812
Cabiscol E, Tamarit J, Ros J (2000). Oxidative stress in bacteria and protein damage by reactive oxygen species. International Microbiology, 3(1): 3–8
Cabrol L, Quéméneur M, Misson B (2017). Inhibitory effects of sodium azide on microbial growth in experimental resuspension of marine sediment. Journal of Microbiological Methods, 133: 62–65
Carmichael S K, Bräuer S L (2015). Microbial diversity and manganese cycling: a review of manganese oxidizing microbial cave communities. In: Summers Engel A, ed. Microbial Life of Cave Systems. Berlin: De Gruyter
Cebrián G, Condón S, Mañas P (2017). Physiology of the inactivation of vegetative bacteria by thermal treatments: mode of action, influence of environmental factors and inactivation kinetics. Foods, 6(12): 107
Cui H, Li B, Li Z, Li X, Xu S (2018). Z-scheme based CdS/CdWO4 heterojunction visible light photocatalyst for dye degradation and hydrogen evolution. Applied Surface Science, 455: 831–840
Diaz J M, Hansel C M, Voelker B M, Mendes C M, Andeer P F, Zhang T (2013). Widespread production of extracellular superoxide by heterotrophic bacteria. Science, 340(6137): 1223–1226
Ding R, Yan W, Wu Y, Xiao Y, Gang H, Wang S, Chen L, Zhao F (2018). Light-excited photoelectrons coupled with bio-photocatalysis enhanced the degradation efficiency of oxytetracycline. Water Research, 143: 589–598
Duckworth O W, Sposito G (2005). Siderophore-manganese(III) interactions. I. Air-oxidation of manganese(ll) promoted by desferrioxamine B. Environmental Science & Technology, 39(16): 6037–6044
Forrez I, Carballa M, Verbeken K, Vanhaecke L, Schlüsener M, Ternes T, Boon N, Verstraete W (2010). Diclofenac oxidation by biogenic manganese oxides. Environmental Science & Technology, 44(9): 3449–3454
Geszvain K, Smesrud L, Tebo B M (2016). Identification of a third Mn (II) oxidase enzyme in Pseudomonas putida GB-1. Applied and Environmental Microbiology, 82(13): 3774–3782
Halstead F D, Thwaite J E, Burt R, Laws T R, Raguse M, Moeller R, Webber M A, Oppenheim B A (2016). Antibacterial activity of blue light against nosocomial wound pathogens growing planktonically and as mature biofilms. Applied and Environmental Microbiology, 82(13): 4006–4016
Hansard S P, Easter H D, Voelker B M (2011). Rapid reaction of nanomolar Mn(II) with superoxide radical in seawater and simulated freshwater. Environmental Science & Technology, 45(7): 2811–2817
Hansel C M, Diaz J M (2021). Production of extracellular reactive oxygen species by marine biota. Annual Review of Marine Science, 13(1): 177–200
Hansel C M, Zeiner C A, Santelli C M, Webb S M (2012). Mn(II) oxidation by an ascomycete fungus is linked to superoxide production during asexual reproduction. Proceedings of the National Academy of Sciences of the United States of America, 109(31): 12621–12625
Hayyan M, Hashim M A, AlNashef I M (2016). Superoxide ion: generation and chemical implications. Chemical Reviews, 116(5): 3029–3085
Hou H, Liu H, Gao F, Shang M, Wang L, Xu L, Wong W, Yang W (2018). Packaging BiVO4 nanoparticles in ZnO microbelts for efficient photoelectrochemical hydrogen production. Electrochimica Acta, 283: 497–508
Huang J, Zhang H (2020). Redox reactions of iron and manganese oxides in complex systems. Frontiers of Environmental Science & Engineering, 14(5): 76
Jung H, Taillefert M, Sun J, Wang Q, Borkiewicz O J, Liu P, Yang L, Chen S, Chen H, Tang Y (2020). Redox cycling driven transformation of layered manganese oxides to tunnel structures. Journal of the American Chemical Society, 142(5): 2506–2513
Koblížek M, Béjà O, Bidigare R R, Christensen S, Benitez-Nelson B, Vetriani C, Kolber M K, Falkowski P G, Kolber Z S (2003). Isolation and characterization of Erythrobacter sp. strains from the upper ocean. Archives of Microbiology, 180(5): 327–338
Kumar A, Ghate V, Kim M J, Zhou W, Khoo G H, Yuk H G (2016). Antibacterial efficacy of 405, 460 and 520 nm light emitting diodes on Lactobacillus plantarum, Staphylococcus aureus and Vibrio parahaemolyticus. Journal of Applied Microbiology, 120(1): 49–56
Learman D R, Voelker B M, Madden A S, Hansel C M (2013). Constraints on superoxide mediated formation of manganese oxides. Frontiers in Microbiology, 4: 262
Learman D R, Voelker B M, Vazquez-Rodriguez A I, Hansel C M (2011). Formation of manganese oxides by bacterially generated superoxide. Nature Geoscience, 4(2): 95–98
Li Y, Chen L, Tian X, Lin L, Ding R, Yan W, Zhao F (2021). Functional role of mixed-culture microbe in photocatalysis coupled with biodegradation: Total organic carbon removal of ciprofloxacin. Science of the Total Environment, 784: 147049
Morris J J, Rose A L, Lu Z (2022). Reactive oxygen species in the world ocean and their impacts on marine ecosystems. Redox Biology, 52: 102285
Murray K J, Webb S M, Bargar J R, Tebo B M (2007). Indirect oxidation of Co(II) in the presence of the marine Mn(II)-oxidizing bacterium Bacillus sp. strain SG-1. Applied and Environmental Microbiology, 73(21): 6905–6909
Nakama K, Medina M, Lien A, Ruggieri J, Collins K, Johnson H A (2014). Heterologous expression and characterization of the manganese-oxidizing protein from Erythrobacter sp. strain SD21. Applied and Environmental Microbiology, 80(21): 6837–6842
Nealson K H (2006). Volume 5: Proteobacteria: Alpha and Beta Subclasses. In: Dworkin M, Falkow S, Rosenberg E, Schleifer K H, Stackebrandt E, editors. The Prokaryotes. New York: Springer, 222–231
Nesbitt H W, Banerjee D (1998). Interpretation of XPS Mn(2p) spectra of Mn oxyhydroxides and constraints on the mechanism of MnO2 precipitation. American Mineralogist, 83(3–4): 305–315
Nico P S, Anastasio C, Zasoski R J (2002). Rapid photo-oxidation of Mn(II) mediated by humic substances. Geochimica et Cosmochimica Acta, 66(23): 4047–4056
Palermo C, Dittrich M (2016). Evidence for the biogenic origin of manganese-enriched layers in Lake Superior sediments. Environmental Microbiology Reports, 8(2): 179–186
Queiroz P S, Ruas F A D, Barboza N R, de Castro Borges W, Guerra-Sá R (2018). Alterations in the proteomic composition of Serratia marcescens in response to manganese (II). BMC Biotechnology, 18(1): 83
Ramadoss G, Suriyaraj S P, Sivaramakrishnan R, Pugazhendhi A, Rajendran S (2021). Mesoporous ferromagnetic manganese ferrite nanoparticles for enhanced visible light mineralization of azoic dye into nontoxic by-products. Science of the Total Environment, 765: 142707
Richardson L L, Aguilar C, Nealson K H (1988). Manganese oxidation in pH and O2 microenvironments produced by phytoplankton. Limnology and Oceanography, 33(3): 352–363
Song Y, Jiang J, Ma J, Zhou Y, von Gunten U (2019). Enhanced transformation of sulfonamide antibiotics by manganese(IV) oxide in the presence of model humic constituents. Water Research, 153: 200–207
Shi Y, Dai Y, Liu Z, Nie X, Zhao S, Zhang C, Jia H (2020). Light-induced variation in environmentally persistent free radicals and the generation of reactive radical species in humic substances. Frontiers of Environmental Science & Engineering, 14(6): 106
Sutherland K M, Coe A, Gast R J, Plummer S, Suffridge C P, Diaz J M, Bowman J S, Wankel S D, Hansel C M (2019). Extracellular superoxide production by key microbes in the global ocean. Limnology and Oceanography, 64(6): 2679–2693
Sutherland K M, Wankel S D, Hansel C M (2020). Dark biological superoxide production as a significant flux and sink of marine dissolved oxygen. Proceedings of the National Academy of Sciences of the United States of America, 117(7): 3433–3439
Tardu M, Bulut S, Kavakli I H (2017). MerR and ChrR mediate blue light induced photo-oxidative stress response at the transcriptional level in Vibrio cholerae. Scientific Reports, 7(1): 40817
Tebo B M, Bargar J R, Clement B G, Dick G J, Murray K J, Parker D, Verity R, Webb S M (2004). Biogenic manganese oxides: properties and mechanisms of formation. Annual Review of Earth and Planetary Sciences, 32(1): 287–328
Tebo B M, Johnson H A, McCarthy J K, Templeton A S (2005). Geomicrobiology of manganese(II) oxidation. Trends in Microbiology, 13(9): 421–428
Tournassat C, Charlet L, Bosbach D, Manceau A (2002). Arsenic(III) oxidation by birnessite and precipitation of manganese(II) arsenate. Environmental Science & Technology, 36(3): 493–500
Wu L, Zhu G, Zhang X, Si Y (2020). Silver nanoparticles inhibit denitrification by altering the viability and metabolic activity of Pseudomonas stutzeri. Science of the Total Environment, 706: 135711
Xia W, Chen R, Li Y, Gao P, Li C, Jin T, Ma J, Ma T (2021). Photo-driven heterogeneous microbial consortium reducing CO2 to hydrocarbons fuel. Journal of Cleaner Production, 326: 129397
Xu J, Zhao M, Pei L, Liu X, Wei L, Li A, Mei Y, Xu Q (2020). Effects of heavy metal mixture exposure on hematological and biomedical parameters mediated by oxidative stress. Science of the Total Environment, 705: 134865
Xu X, Li Y, Li Y, Lu A, Qiao R, Liu K, Ding H, Wang C (2019). Characteristics of desert varnish from nanometer to micrometer scale: a photo-oxidation model on its formation. Chemical Geology, 522:55–70
Yang F, Zheng Y, Tian X, Liu Y, Li J, Shao Z, Zhao F (2021). Redox cycling of manganese by Bacillus horikoshii biET1 via oxygen switch. Electrochimica Acta, 375: 137963
Yang G, Ni W, Wu K, Wang H, Yang B E, Jia X, Jin R (2013). The effect of Cu(II) stress on the activity, performance and recovery on the Anaerobic Ammonium-Oxidizing (Anammox) process. Chemical Engineering Journal, 226: 39–45
Yang X, Peng Q, Liu L, Tan W, Qiu G, Liu C, Dang Z (2020). Synergistic adsorption of Cd(II) and As(V) on birnessite under electrochemical control. Chemosphere, 247: 125822
Zhang T, Liu L, Tan W, Suib S L, Qiu G, Liu F (2018). Photochemical formation and transformation of birnessite: effects of cations on micromorphology and crystal structure. Environmental Science & Technology, 52(12): 6864–6871
Zhao H, Wang Y, Liu Y, Yin K, Wang D, Li B, Yu H, Xing M (2021). ROS-induced hepatotoxicity under cypermethrin: involvement of the crosstalk between Nrf2/Keap1 and NF-kappaB/ikappaB-alpha pathways regulated by proteasome. Environmental Science & Technology, 55(9): 6171–6183
Zheng Y, Kappler A, Xiao Y, Yang F, Mahadeva G D, Zhao F (2019). Redox-active humics support interspecies syntrophy and shift microbial community. Science China Technological Sciences, 62(10): 1695–1702
Zhou H, Fu C (2020). Manganese-oxidizing microbes and biogenic manganese oxides: characterization, Mn(II) oxidation mechanism and environmental relevance. Reviews in Environmental Science and Biotechnology, 19(3): 489–507
Acknowledgements
This work was supported by the National Natural Science Foundation of China (Nos. 42021005 and 22025603).
Author information
Authors and Affiliations
Corresponding author
Additional information
Highlights
• Term of manganese-oxidizing microorganisms should be reconsidered.
• Visible light induces heterotrophic bacteria to produce superoxide.
• Heterotrophic bacteria oxidize Mn(II) ions with a fast oxidation rate.
• Superoxide oxidizing Mn(II) ions is an unintended side reaction of bacteria.
• Superoxide is an important oxidation force of Mn(II) in the environment.
Supporting Information
Rights and permissions
About this article
Cite this article
Yang, F., Li, J., Wang, H. et al. Visible light induces bacteria to produce superoxide for manganese oxidation. Front. Environ. Sci. Eng. 17, 19 (2023). https://doi.org/10.1007/s11783-023-1619-y
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11783-023-1619-y