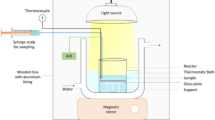
Abstract
Due to the low water solubility of polybrominated diphenyl ethers, organic solvent is usually added into the oxidation system to enhance the removal efficiency. In this study the photocatalytic degradation of decabromodiphenyl ether (BDE209), a type of polybrominated diphenyl ether used throughout the world, in pure water without the addition of organic solvent was investigated. In the pure water system, BDE209 was not dissolved but dispersed as nano-scale particles with a mean diameter of 166 nm. Most of BDE209 (>98%) were removed within 4 h and the final debromination ratio was greater than 80%. Although the addition of organic solvent (tetrahydrofuran, THF) could lead to a relatively high BDE209 degradation rate, the final debromination ratio (<50%) was much lower than that in pure water system. Major oxidation intermediates of tetrahydrofuran, including tetrahydro-2-furanol and γ-butyrolactone, were detected indicating the engagement of THF in the BDE209 degradation process. The photocatalytic degradation of BDE209 in the pure water system followed first-order kinetics. The BDE209 degradation rate constant increased from 0.0011 to 0.0023 min−1 as the pH increased from 3 to 9.
Similar content being viewed by others
References
Ahn M Y, Filley T R, Jafvert C T, Nies L, Hua I, Bezares-Cruz J. Photodegradation of decabromodiphenyl ether adsorbed onto clay minerals, metal oxides, and sediment. Environmental Science & Technology, 2006, 40(1): 215–220
Hites R A. Polybrominated diphenyl ethers in the environment and in people: a meta-analysis of concentrations. Environmental Science & Technology, 2004, 38(4): 945–956
Hutzinger O, Thoma H. Polybrominated dibenzodioxins and dibenzofurans: the flame retardant issue. Chemosphere, 1987, 16(8–9): 1877–1880
Watanabe I, Kashimoto T, Tatsukawa R. Polybrominated diphenyl ethers in marine fish, shellfish and river and marine sediments in Japan. Chemosphere, 1987, 16(10–12): 2389–2396
Covaci A, Voorspoels S, de Boer J. Determination of brominated flame retardants, with emphasis on polybrominated diphenyl ethers (PBDEs) in environmental and human samples—a review. Environment International, 2003, 29(6): 735–756
Mai B, Chen S, Luo X, Chen L, Yang Q, Sheng G, Peng P, Fu J, Zeng E Y. Distribution of polybrominated diphenyl ethers in sediments of the Pearl River Delta and adjacent South China Sea. Environmental Science & Technology, 2005, 39(10): 3521–3527
Schecter A, Päpke O, Tung K C, Staskal D, Birnbaum L. Polybrominated diphenyl ethers contamination of United States food. Environmental Science & Technology, 2004, 38(20): 5306–5311
Luross J M, Alaee M, Sergeant D B, Cannon C M, Michael Whittle D, Solomon K R, Muir D C G. Spatial distribution of polybrominated diphenyl ethers and polybrominated biphenyls in lake trout from the Laurentian Great Lakes. Chemosphere, 2002, 46(5): 665–672
Darnerud P O, Eriksen G S, Jóhannesson T, Larsen P B, Viluksela M. Polybrominated diphenyl ethers: occurrence, dietary exposure, and toxicology. Environmental Health Perspectives, 2001, 109(s1 Suppl 1): 49–68
La Guardia M J, Hale R C, Harvey E. Detailed polybrominated diphenyl ether (PBDE) congener composition of the widely used penta-, octa-, and deca-PBDE technical flame-retardant mixtures. Environmental Science & Technology, 2006, 40(20): 6247–6254
Eriksson J, Green N, Marsh G, Bergman A. Photochemical decomposition of 15 polybrominated diphenyl ether congeners in methanol/water. Environmental Science & Technology, 2004, 38(11): 3119–3125
Zhao H, Zhang F, Qu B, Xue X, Liang X. Wet air co-oxidation of decabromodiphenyl ether (BDE209) and tetrahydrofuran. Journal of Hazardous Materials, 2009, 169(1–3): 1146–1149
Moreira Bastos P, Eriksson J, Vidarson J, Bergman A. Oxidative transformation of polybrominated diphenyl ether congeners (PBDEs) and of hydroxylated PBDEs (OH-PBDEs). Environmental Science and Pollution Research International, 2008, 15(7): 606–613
Bastos P M, Eriksson J, Bergman A. Photochemical decomposition of dissolved hydroxylated polybrominated diphenyl ethers under various aqueous conditions. Chemosphere, 2009, 77(6): 791–797
Hardy ML. A comparison of the properties of the major commercial PBDPO/PBDE product to those of major PBB and PCB products. Chemosphere, 2002, 46(5): 717–728
Sun C, Zhao D, Chen C, Ma W, Zhao J. TiO2-mediated photocatalytic debromination of decabromodiphenyl ether: kinetics and intermediates. Environmental Science & Technology, 2009, 43(1): 157–162
Lacorte S, Guillamon M. Validation of a pressurized solvent extraction and GC-NCI-MS method for the low level determination of 40 polybrominated diphenyl ethers in mothers’ milk. Chemosphere, 2008, 73(1): 70–75
Henry T B, Menn FM, Fleming J T, Wilgus J, Compton R N, Sayler G S. Attributing effects of aqueous C60 nano-aggregates to tetrahydrofuran decomposition products in larval zebrafish by assessment of gene expression. Environmental Health Perspectives, 2007, 115(7): 1059–1065
Robertson A. Tetrahydrofuran hydroperoxide. Nature, 1948, 162(4108): 153
Murai S, Sonoda N, Tsutsumi S. Redox reaction of tetrahydrofuran hydroperoxide. Bulletin of the Chemical Society of Japan, 1963, 36(5): 527–530
Sun C, Chang W, Ma W, Chen C, Zhao J. Photoreductive debromination of decabromodiphenyl ethers in the presence of carboxylates under visible light irradiation. Environmental Science & Technology, 2013, 47(5): 2370–2377
Yin L, Niu J, Shen Z, Chen J. Mechanism of reductive decomposition of pentachlorophenol by Ti-doped β-Bi(2)O(3) under visible light irradiation. Environmental Science & Technology, 2010, 44(14): 5581–5586
Akpan U G, Hameed B H. Parameters affecting the photocatalytic degradation of dyes using TiO2-based photocatalysts: a review. Journal of Hazardous Materials, 2009, 170(2–3): 520–529
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Zhang, M., Lu, J., He, Y. et al. Photocatalytic degradation of polybrominated diphenyl ethers in pure water system. Front. Environ. Sci. Eng. 10, 229–235 (2016). https://doi.org/10.1007/s11783-014-0762-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11783-014-0762-x