Abstract
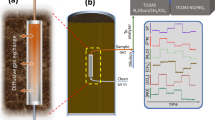
Development and demonstration of reliable measurement techniques that can detect and help quantify the nature and extent of elemental mercury (Hg(0)) in the subsurface are needed to reduce uncertainties in the decision-making process and increase the effectiveness of remedial actions. We conducted field tests at the Y-12 National Security Complex in Oak Ridge, Tennessee, USA, to determine if sampling and analysis of Hg(0) vapors in the shallow subsurface (< 0.3 m depth) can be used to as an indicator of the location and extent of Hg(0) releases in the subsurface. We constructed a rigid polyvinyl chloride push probe assembly, which was driven into the ground. Soil gas samples were collected through a sealed inner tube of the assembly and were analyzed immediately in the field with a Lumex and/or Jerome Hg(0) analyzer. Time-series sampling showed that Hg vapor concentrations were fairly stable over time, suggesting that the vapor phase Hg(0) was not being depleted and that sampling results were not sensitive to the soil gas purge volume. Hg(0) vapor data collected at over 200 push probe locations at 3 different release sites correlated very well to areas of known Hg(0) contamination. Vertical profiling of Hg(0) vapor concentrations conducted at two locations provided information on the vertical distribution of Hg(0) contamination in the subsurface. We conclude from our studies that soil gas sampling and analysis can be conducted rapidly and inexpensively at large scales to help identify areas contaminated with Hg(0).
Similar content being viewed by others
References
Blackstone Institute produced in collaboration with Green Cross Switerzland. The World’s Worst Pollution Problems: Assessing Health Risks at Hazardous Waste Sites, 2012. Available at http://www.worstpolluted.org/
Brooks S C, Southworth G R. History of mercury use and environmental contamination at the Oak Ridge Y-12 Plant. Environmental Pollution, 2011, 159(1): 219–228
Peterson M J, Looney B B, Southworth G R, Eddy-Dilek C, Watson D, Ketelle R, Bogle M A. Conceptual model of primary mercury source, transport pathways, and flux at the Y-12 complex and upper East Fork Poplar Creek, Oak Ridge, Tennessee. ORNL, U. S. Atomic Energy Commission, 2011, TM-2011 (75)
United Nations Environment Programme. Global mercury assessment, chemicals report. UNEP Chemicals, Geneva Switzerland, 2002
ChemRisk. Mercury releases from Lithium enrichment at the Oak Ridge Y-12 Plant -A reconstruction of historical releases and off-site doses and health risks. Task 2 Report of the Oak Ridge Dose Reconstruction, Vol. 2. Tennessee Department of Health, 1999
US Department of Energy. Major risk factors, Integrated Facility Disposition Project (IFDP), Oak Ridge, Tenn. External Technical Review (ETR) Report, 2008
Thompson G, Marrin D. A dynamic approach. Ground Water Monitoring Review, 1987, 7(3): 88–93
Ballestrero T, Herzog B, Thompson G. Monitoring and sampling the vadose zone. In: Nielsen D M, ed, Practical Handbook of Ground-Water Monitoring. Chelsea, Michigan: Lewis Publishers, Inc. 2006, 207–247
US Environmental Protection Agency. Guidance document for soilgas surveying, prepared under EPA EMSL-LV Contract No. 68-03-3245 by C. L. Mayer, Lockheed Engineering and Sciences Company, Las Vegas, N.V. (in press)
US Environmental Protection Agency. Final project report for the development of an active soil gas sampling method. EPA/600/R-07/076. Office of Research and Development, National Exposure Research Laboratory, Las Vegas, N.V., 2007
Johnson D W, Benesch J A, Gustin M S, Schorran D S, Lindberg S E, Coleman J S. Experimental evidence against diffusion control of Hg evasion from soils. Science of the Total Environment, 2003, 304(1–3): 175–184
Moore C W, Castro M S. Investigation of factors affecting gaseous mercury concentrations in soils. Science of the Total Environment, 2012, 419: 136–143
Moore CW, Castro MS, Brooks S B. A simple and accurate method to measure total gaseous mercury concentrations in unsaturated soils. Water, Air, and Soil Pollution, 2011, 218(1–4): 3–9
Sigler J M, Lee X. Gaseous mercury in background forest soil in the northeastern United States. Journal of Geophysical Research-Biogeosciences, 2006, 111
Wallschlager D, Kock H H, Schroeder W H, Lindberg S E, Ebinghaus R, Wilken R D. Estimating gaseous mercury emissions from contaminated floodplain soils to the atmosphere with simple field measurement techniques. Water, Air, and Soil Pollution, 2002, 135(1/4): 39–54
Kriger A A, Turner R R. Field analysis of mercury in water, sediment and soil using static headspace analysis. Water, Air, and Soil Pollution, 1995, 80(1–4): 1295–1304
Carlton H W, Price V, Cook J R. Mercury in shallow Savannah River Plant soil. DPST-86-314. Westinghouse Savannah River Co., Aiken, SC. 1988.
Wang G, Chenglong L, Wang J, Liu W, Zhang P. The use of soil mercury and radon gas surveys to assist the detection of concealed faults in Fuzhou City, China. Environmental Geology, 2006, 51(1): 83–90
Kromera E, Friedricha G, Wallnera P. Mercury and mercury compounds in surface air, soil gas, soils and rocks. Journal of Geochemical Exploration, 1981, 15(1–3): 51–62
Miller C L, Watson D B, Lester B P, Lowe K A, Pierce E M, Liang L. Characterization of soils from an industrial complex contaminated with elemental mercury. Environmental Research, 2013, 125: 20–29
Rothschild E R, Turner R R, Stow S H, Bogle M A, Hyder L K, Sealand O M, Wyrick H J. Investigation of subsurface mercury at the Oak Ridge Y-12 Plant. ORNL/TM-9092. Oak Ridge National Laboratory, Oak Ridge, Tenn., 1984
Turner R R, Kamp G E, Bogle M A, Switek J, McElhaney R. Sources and discharges of mercury in drainage waters at the Oak Ridge Y-12 Plant. Y/TS-90. Oak Ridge Y-12 Plant, Oak Ridge, Tenn. 1985
Oak Ridge Institute for Science and Education. Characterization report for the 81-10 area in the Upper East Fork Poplar Creek Area at the Oak Ridge Y-12 National Security Complex, Oak Ridge, Tenn. DOE/OR/01-2485&D1, Oak Ridge, Tenn., 2010
Arizona Instrument L L C. Jerome® 431-X Mercury Vapor Analyzer, Operations Manual. 3375 N Delaware Street, Chandler, Ariz, 85225, 2009
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Watson, D., Miller, C., Lester, B. et al. Mercury source zone identification using soil vapor sampling and analysis. Front. Environ. Sci. Eng. 9, 596–604 (2015). https://doi.org/10.1007/s11783-014-0709-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11783-014-0709-2