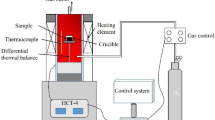
Abstract
Beach titanomagnetite (TTM) provides a cheap alternative source of Fe and Ti, but this ore is difficult to process to make suitable concentrates for the blast furnace. Recently studies showed that it is feasible to separate Fe and Ti by coal-based direct reduction. In this study, beach TTM was selected as the research object, the effects of reducing agents on reducing atmosphere in coal-based direct reduction of beach TTM were analyzed, and the role of volatiles was also studied. The results showed that when bitumite and coke were used as reducing agents of TTM, the CO produced from volatiles was involved in the reduction reaction, and the generated CO2 provided the raw material for the reaction of TTM. The reduction effect of bitumite was better than that of coke. The reason is that bitumite+TTM had a higher gas generation rate and produced a higher CO partial pressure, while coke+TTM had a lower gas generation rate and produced a lower CO partial pressure. When graphite was used as a reducing agent, there was a solid-solid reaction in the early stage in the reaction. With the continuous accumulation of CO2, the Boudouad reaction started and accelerated. Graphite+TTM also produced a higher CO partial pressure.
摘要
海滨钛磁铁矿资源储量丰富,是钛、铁资源的重要来源,但是采用常规选矿方法难以得到用于高炉冶炼的铁精矿。研究表明,采用煤基直接还原工艺可以实现钛磁铁矿的钛铁分离。本文研究了还原剂对钛磁铁矿煤基还原过程中还原气氛的影响,并分析了挥发分的作用。结果表明,当烟煤和焦炭作为钛磁铁矿的还原剂时,产生的CO参与了还原反应,生成的CO2进一步作为碳热还原反应所需要的原料。同时发现烟煤的还原效果优于焦炭,这主要是因为烟煤与钛磁铁矿混合物具有较高的气体生成速率,可以产生较高的CO分压值,而焦炭与钛磁铁矿混合物具有较低的气体生成速率,导致产生的CO分压值较低。当石墨作为还原剂时产生和烟煤相似的还原效果,在反应的早期阶段存在固-固反应,随着CO2的不断累积,Boudouad开始反应并加速,产生了较高的CO分压值。
Similar content being viewed by others
References
BRATHWAITE R L, GAZLEY M F, CHRISTIE A B. Provenance of titanomagnetite in ironsands on the west coast of the North Island, New Zealand [J]. Journal of Geochemical Exploration, 2017, 178: 23–34. DOI: https://doi.org/10.1016/j.gexplo.2017.03.013.
ZULHAN Z, ADHIWIGUNA I B G S, FUADI A, et al. Solid-state reduction of an Indonesian iron sand concentrate using subbituminous coal [J]. Canadian Metallurgical Quarterly, 2021, 60(1): 12–20. DOI: https://doi.org/10.1080/00084433.2021.1918508.
WANG Zhe, PINSON D, CHEW S, et al. Interaction of new Zealand ironsand and flux materials [J]. ISIJ International, 2016, 56(8): 1315–1324. DOI: https://doi.org/10.2355/isijinternational.isijint-2015-728.
WANG Zhen-yang, ZHANG Jian-liang, LIU Zheng-jian, et al. Formation of multiple microstructures during the reduction of ironsand [J]. JOM, 2019, 71(5): 1776–1784. DOI: https://doi.org/10.1007/s11837-018-3279-0.
JENA M S, TRIPATHY H K, MOHANTY J K, et al. Roasting followed by magnetic separation: A process for beneficiation of titano-magnetite ore [J]. Separation Science and Technology, 2015, 50(8): 1221–1229. DOI: https://doi.org/10.1080/01496395.2014.965834.
LONGBOTTOM R J, MONAGHAN B J, MATHIESON J G. Development of a bonding phase within titanomagnetite-coal compacts [J]. ISIJ International, 2013, 53(7): 1152–1160. DOI: https://doi.org/10.2355/isijinternational.53.1152.
GENG Chao, SUN Ti-chang, YANG Hui-fen, et al. Effect of Na2SO4 on the embedding direct reduction of beach titanomagnetite and the separation of titanium and iron by magnetic separation [J]. ISIJ International, 2015, 55(12): 2543–2549. DOI: https://doi.org/10.2355/isijinternational.isijint-2015-420.
ZHAO Yong-qiang, SUN Ti-chang, ZHAO Hong-yu, et al. Effect of MgO and CaCO3 as additives on the reduction roasting and magnetic separation of beach titanomagnetite concentrate [J]. ISIJ International, 2019, 59(6): 981–987. DOI: https://doi.org/10.2355/isijinternational.isijint-2018-757.
HU Tian-yang, SUN Ti-chang, KOU Jue, et al. Recovering titanium and iron by co-reduction roasting of seaside titanomagnetite and blast furnace dust [J]. International Journal of Mineral Processing, 2017, 165: 28–33. DOI: https://doi.org/10.1016/j.minpro.2017.06.003.
ZHAO Yong-qiang, SUN Ti-chang, ZHAO Hong-yu, et al. Effect of reductant type on the embedding direct reduction of beach titanomagnetite concentrate [J]. International Journal of Minerals, Metallurgy, and Materials, 2019, 26(2): 152–159. DOI: https://doi.org/10.1007/s12613-019-1719-7.
WANG Ming-yu, ZHOU Sheng-fan, WANG Xue-wen, et al. Recovery of iron from chromium vanadium-bearing titanomagnetite concentrate by direct reduction [J]. JOM, 2016, 68(10): 2698–2703. DOI: https://doi.org/10.1007/s11837-016-2083-y.
XU Cheng-yan, SUN Ti-chang, QI Chao-ying, et al. Effects of coal types on direct reduction and dephosphorization synchronously of high-phosphorus oolitic hematite [J]. Metal Mine, 2010(8): 46–50. (in chinese)
LI Xiao-hui, KOU Jue, SUN Ti-chang, et al. Coal and coke based reduction of vanadium titanomagenetite concentrate by the addition of calcium carbonate [J]. Mineral Processing and Extractive Metallurgy Review, 2021, 42(2): 115–122. DOI: https://doi.org/10.1080/08827508.2019.1702039.
SUN Hao-yan, DONG Xiang-juan, SHE Xue-feng, et al. Solid state reduction of titanomagnetite concentrate by graphite [J]. ISIJ International, 2013, 53(4): 564–569. DOI: https://doi.org/10.2355/isijinternational.53.564.
HU Tu, LV Xue-wei, BAI Chen-guang, et al. Reduction behavior of Panzhihua titanomagnetite concentrates with coal [J]. Metallurgical and Materials Transactions B, 2013, 44(2): 252–260. DOI: https://doi.org/10.1007/s11663-012-9783-7.
de CARVALHO R J, QUARIGUASI NETTO P G, D’ABREU J C. Kinetics of reduction of composite pellets containing iron ore and carbon [J]. Canadian Metallurgical Quarterly, 1994, 33(3): 217–225. DOI: https://doi.org/10.1179/cmq.1994.33.3.217.
ZHAO Yong-qiang, SUN Ti-chang, WANG Zhe. Extraction of iron from refractory titanomagnetite by reduction roasting and magnetic separation [J]. ISIJ International, 2021, 61(1): 93–99. DOI: https://doi.org/10.2355/isijinternational.isijint-2020-251.
JUNG S M. Effects of CaO/CaCO3 on the carbothermic reduction of titanomagnetite ores [J]. Metallurgical and Materials Transactions B, 2015, 46(3): 1162–1174. DOI: https://doi.org/10.1007/s11663-015-0341-y.
Author information
Authors and Affiliations
Corresponding author
Additional information
Foundation item
Project(52104257) supported by the National Natural Science Foundation of China
Contributors
SUN Ti-chang developed the overarching research goals. ZHAO Yong-qiang conducted the literature review and wrote the manuscript. ZHOU Wen-tao validated the proposed method with practical experiments and wrote the first draft of the manuscript. ASADULLAH Ahmadzai edited the manuscript. LYU Xian-jun directed the revision of the paper
Conflict of interest
ZHAO Yong-qiang, ZHOU Wen-tao, LYU Xian-jun and SUN Ti-chang declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Zhao, Yq., Zhou, Wt., Lyu, Xj. et al. Effect of reducing agents on reducing atmosphere in coal-based direct reduction of beach titanomagnetite. J. Cent. South Univ. 29, 3670–3677 (2022). https://doi.org/10.1007/s11771-022-5177-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11771-022-5177-4
Key words
- beach titanomagnetite
- coal-based direct reduction
- magnetic separation
- reducing agents
- reducing atmosphere