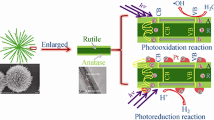
Abstract
Photocatalytic carbon dioxide reduction reaction (CO2RR) has been considered as one of most effective ways to solve the current energy crisis and environmental problems. However, the practical application of photocatalytic CO2RR is largely hindered by lock of efficient catalyst. Here, hierarchical titanium dioxide (TiO2) nanostructures with a highly active {001} surface were successfully synthesized by a facile approach from metal Ti powders. The obtained hierarchical TiO2 nanostructures were composed of TiO2 nanorods, which have a diameter about 5–10 nm and a length of several micrometers. It is found that these nanorods have exposed {001} facets. On the other hand, these hierarchical TiO2 nanostructures have a good light-harvesting efficiency with the help of TiO2 nanorods component and large specific surface area. Therefore, these hierarchical TiO2 nanostructures exhibit a much better activity for photocatalytic CO2 reduction than that of commercial TiO2 (P25). This high activity can be attributed to the synergistic effects of active surface, efficient charge transfer along nanorods and good light harvesting in the nanorod-hierarchical nanostructures.
摘要
光催化二氧化碳还原被认为是能够同时解决能源和环境问题的最有效方式之一。但高效的二氧 化碳还原催化剂的缺乏限制其实际应用。在本文中,我们成功合成了具有{001}高活性晶面的分级二 氧化钛纳米棒结构,纳米棒的直径为5~10 nm,长度为几个微米。分级纳米棒结构使得其具有更大的 比表面积,进而大大促进了光吸收。具有有效电荷传输、更大比表面积及更强光吸收的分级结构二氧 化钛纳米棒与商业的P25 二氧化钛相比具有更强的二氧化碳光催化还原性能。
Similar content being viewed by others
References
LINSEBIGLER A L, LU G Q, YATES J T. Photocatalysis on TiO2 surfaces: Principles, mechanisms, and selected results [J]. Chem Rev, 1995, 95: 735–758.
HE H N, GAN Q M, WANG H Y, XU G L, ZHANG X Y, HUANG D, FU F, TANG Y G, AMINE K, SHAO M H. Structure-dependent performance of TiO2/C as anode material for Na-ion batteries [J]. Nano Energy, 2018, 44: 217–227.
ZHANG Q, HE H N, HUANG X B, YAN J, TANG Y G, WANG H Y. TiO2@C nanosheets with highly exposed (001) facets as a high-capacity anode for Na-ion batteries [J]. Chem Eng J, 2018, 332: 57–65.
XUE X, SUN D, ZENG X G, HUANG X B, ZHANG H H, TANG Y G, WANG H Y. Two-step carbon modification of NaTi2(PO4)3 with improved sodium storage performance for Na-ion batteries [J]. Journal of Central South University, 2018, 25(10): 2320–2331.
LIU A Q, LIU K, ZHOU H M, LI H M, QIU X Q, YANG Y, LIU M. Solution evaporation processed high quality perovskite films [J]. Sci Bull, 2018, 63: 1591–1596.
CENTI G, PERATHONER S. Opportunities and prospects in the chemical recycling of carbon dioxide to fuels [J]. Catal Today, 2009, 148: 191–205.
PAN J, LIU G, LU G Q, CHENG H M. On the true Photoreactivity Order of {001}, {010}, and {101} facets of anatase TiO2 crystals [J]. Angew Chem Int Ed, 2011, 50: 2133–2137.
ROY N, SOHN Y, PRADHAN D. Synergy of low-energy {101} and high-energy {001} TiO2 crystal facets for enhanced photocatalysis [J]. ACS Nano, 2013, 7: 2532–2540.
LAZZERI M, VITTADINI A, SELLONI A. Erratum: Structure and energetics of stoichiometric TiO2 anatase surfaces [Phys. Rev. B 63, 155409 (2001)] [J]. Phys Rev B, 2002, 65: 119901.
DIEBOLD U. The surface science of titanium dioxide [J]. Surf Sci Rep, 2003, 48: 53–229.
SELLONI A. Fluorine-containing species can cause titania to crystallize with an unusually large fraction of reactive {001} facets [J]. Nat Mater, 2008, 7: 613–615.
YANG H G, SUN C H, QIAO S Z, ZOU J, LIU G, SMITH S C, CHENG H M, LU G Q. Anatase TiO2 single crystals with a large percentage of reactive facets [J]. Nature, 2008, 453: 638–641.
LIU M, PIAO L Y, LU W M, JU S T, ZHAO L, ZHOU C L, YAN Z J, WANG W J. Anatase TiO2 single crystals with exposed {001} and {110} facets facile synthesis and enhanced photocatalysis [J]. Chem Coumm, 2010, 46: 1664–1666.
KAKIHANA M, TADA M, SHIRO M, PETRYKIN V, OSADA M, NAKAMURA Y. Structure and Stability of water soluble (NH4)8[Ti4(C6H4O7)4(O2)4]8H2O [J]. Inorg Chem, 2001, 40: 891–894.
MAO Y B, WONG S S. Size- and shape-dependent transformation of nanosized titanate into analogous anatase titania nanostructures [J]. J Am Chem Soc, 2006, 128: 8217–8226.
AO Y, FU D, YUAN C. A simple method for the preparation of titania hollow sphere [J]. Catal Commun, 2008, 9: 2574–2577.
YU J G, LIU S W, YU H G. Microstructures and photoactivity of mesoporous anatase hollow microspheres fabricated by fluoride-mediated self-transformation [J]. J Catal, 2007, 249: 59–66.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Foundation item: Project(21872174) supported by the National Natural Science Foundation of China; Projects(2017CX003, 20180018050001) supported by the Innovation-Driven Plan in Central South University, China; Project supported by State Key Laboratory of Powder Metallurgy in Central South University, China; Project(JCYJ20180307151313532) supported by Shenzhen Science and Technology Innovation Project, China; Project supported by the Thousand Youth Talents Plan of China; Project supported by the Hundred Youth Talents Program of Hunan, China
Rights and permissions
About this article
Cite this article
Cao, Mq., Liu, K., Zhou, Hm. et al. Hierarchical TiO2 nanorods with a highly active surface for photocatalytic CO2 reduction. J. Cent. South Univ. 26, 1503–1509 (2019). https://doi.org/10.1007/s11771-019-4106-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11771-019-4106-7