Abstract
Purpose
Improvements in breast cancer management continue to increase survival and life expectancy after treatment. Yet the adverse effects of treatment may persist long term, threatening physical, psychological, and social wellbeing, leading to impaired quality of life (QOL). Upper-body morbidity (UBM) such as pain, lymphoedema, restricted shoulder range of motion (ROM), and impaired function are widely reported after breast cancer treatment, but evidence demonstrating its impact on QOL is inconsistent. Therefore, the aim of the study was to conduct a systematic review and meta-analysis evaluating the effect of UBM on QOL following primary breast cancer treatment.
Methods
The study was prospectively registered on PROSPERO (CRD42020203445). CINAHL, Embase, Emcare, PsycInfo, PubMed/Medline, and SPORTDiscus databases were searched for studies reporting QOL in individuals with and without UBM following primary breast cancer treatment. Primary analysis determined the standardised mean difference (SMD) in physical, psychological, and social wellbeing scores between UBM + /UBM − groups. Secondary analyses identified differences in QOL scores between groups, according to questionnaire.
Results
Fifty-eight studies were included, with 39 conducive to meta-analysis. Types of UBM included pain, lymphoedema, restricted shoulder ROM, impaired upper-body function, and upper-body symptoms. UBM + groups reported poorer physical (SMD = − 0.99; 95%CI = − 1.26, − 0.71; p < 0.00001), psychological (SMD = − 0.43; 95%CI = − 0.60, − 0.27; p < 0.00001), and social wellbeing (SMD = − 0.62; 95%CI = − 0.83, − 0.40; p < 0.00001) than UBM − groups. Secondary analyses according to questionnaire showed that UBM + groups rated their QOL poorer or at equal to, UBM − groups across all domains.
Conclusions
Findings demonstrate the significant, negative impact of UBM on QOL, pervading physical, psychological, and social domains.
Implications for Cancer Survivors
Efforts to assess and minimise the multidimensional impact of UBM are warranted to mitigate impaired QOL after breast cancer.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
With the advent of new and effective methods for detecting, diagnosing, and treating breast cancer, life expectancy following the completion of primary treatment is improving [1]. However, adverse cancer and treatment-related effects continue to arise over the course of treatment. If these persist, they stand to threaten physical, psychological, social, and spiritual wellbeing in the long term.
In the case of breast cancer, upper-body treatment modalities that target areas of the breast, chest, and axilla, leaving nearby musculoskeletal, lymphatic and neural structures vulnerable to injury or impairment [2, 3]. Surgery and radiation therapy to the breast and axillary or subclavicular lymph nodes can cause tissue scarring/fibrosis, axillary cording, and muscle tightness, leading to impaired shoulder kinetics, reductions in shoulder range of motion (ROM) [4], and pain or discomfort [5]. Damage to the lymphatic system can result in the development of breast or upper-limb lymphoedema, the accumulation of lymphatic fluid leading to extremity swelling [6, 7]. Nerve damage accrued during local treatment can lead to neuropathic pain, paraesthesia, and altered muscle activation [8, 9]. Systemic treatment is also implicated in the development of upper-body symptoms. Neurotoxic chemotherapy can induce peripheral neuropathy and manifest as pain or altered sensation in the distal extremities. Hormone therapies are known to cause arthralgia and myalgia, which may be experienced in the joints and muscles of the upper limb [10].
Treatment-related upper-body concerns may be acute, resolving with time after treatment [11, 12]. However, up to 51% of individuals report experiencing at least one upper-body symptom or limitation within 18 months following breast cancer treatment [13] and survivors of up to 10-years post-treatment report the presence of breast cancer-related lymphoedema [14], chronic somatic or neuropathic pain, restricted shoulder ROM, chemotherapy-induced peripheral neuropathy, or a combination of these [14,15,16,17,18].
Due to the prevalence and persistence of treatment-related upper-body morbidity (UBM), it is imperative to understand the impact of UBM on daily functioning and quality of life (QOL) long term, so that it can be suitably addressed [19,20,21,22,23,24]. However, substantial variation exists in the way that UBM is categorized — such as by type, cause, or severity [14] — the time at which UBM and QOL are assessed post-treatment [25], and the domains of QOL that are measured. As a result, the direction and magnitude of the effect of all types of UBM on multiple aspects of one’s life remains unclear. Given the volume and heterogeneity of studies reporting QOL and UBM after breast cancer, a meta-synthesis to elucidate the impact of UBM that persists beyond primary treatment on each domain of QOL is warranted. A greater understanding of the relationship between persisting UBM and QOL will help contribute to improving care provided after breast cancer treatment.
Aim
The aim of this study was to conduct a systematic review and meta-analysis, to evaluate the effect of persistent UBM following primary breast cancer treatment, on multiple domains of QOL.
Methods
The review was conducted in accordance with the PRISMA 2020 statement [26], and the Cochrane handbook for systematic review and meta-analysis [27]. The study was prospectively registered on PROSPERO (CRD42020203445).
CINAHL, Embase, Emcare, PsycInfo, PubMed/Medline, and SPORTDiscus databases were searched without language restrictions, from inception until 25 September 2020. Subject headings and keywords referencing breast cancer, QOL, and treatment-related UBM were employed in the search. A detailed search strategy is included in the supplementary materials (Online resource 1). The database search was repeated on 8 December 2021 and 7 March 2023.
Studies which met the following criteria were eligible for inclusion: (1) published in English language; (2) observational (cross-sectional or longitudinal) or interventional (outcomes of interest assessed prior to delivery of an intervention); (3) sample comprised of individuals who had completed primary treatment for breast cancer of any stage, type, and grade; (4) QOL reported in breast cancer survivors with and without UBM discretely, using validated, multidimensional QOL assessment tools.
Treatment-related UBM was defined as the presence of at least one of any upper-body symptom or limitation arising after breast cancer treatment, indicated by self-report or objective clinical assessment. The “condition” was dichotomised into UBM present (UBM +) or UBM absent (UBM −). Where studies grouped participants into UBM groups more than once—for example, on the basis of an interlimb circumference measure, and on the basis of self-report — QOL data were extracted based on the objective data categorisations of UBM + / − . If multiple UBM + or UBM − groups were present in one study – for example, lymphoedema and reduced shoulder ROM groups – QOL data were combined to create UBM + / − groups using Review Manager v5.4.1 (The Cochrane Collaboration) or provided by authors upon request.
Records were screened for eligibility in two stages and in duplicate. Title and abstract screening [EM (100%); KM (75%); BC (25%)] and full text screening [EM (100%); BC (50%); AH (50%)] were completed using the Rayyan systematic review web application (Rayyan Systems Inc) [28] and COVIDENCE systematic review software (Veritas Health Innovation) [29], respectively. Data from included articles were extracted in duplicate into predetermined spreadsheets by authors EM, BC, and NA. Where studies met inclusion criteria but UBM or QOL data could not be adequately extracted, authors were contacted and followed up via email.
Study quality was assessed in duplicate by EM, BC and NA using the Joanna Briggs Institute (JBI) Critical Appraisal Checklist for Analytical Cross-sectional Studies [30]. The checklist consists of eight criteria for assessing the risk of publication bias in included studies. As per the JBI Manual for Evidence Synthesis [31], reviewers determined a priori that studies which met ≥ 75% of the criteria would be considered “good” quality.
Statistical analysis
Studies which presented QOL data (mean with variance), for UBM + and UBM − groups discretely, were included in the meta-analysis. Where QOL was assessed on multiple occasions, the measure taken at the latest timepoint post-treatment was included to capture the effect of persistent rather than acute UBM on QOL. Where the results of one study were reported across multiple publications, the record with the most complete dataset was included. Meta-analyses were conducted in Review Manager v5.4.1 (The Cochrane Collaboration) [32].
Primary analysis
The primary meta-analyses evaluated the effect of UBM on (1) physical wellbeing, (2) psychological/emotional wellbeing, and (3) social wellbeing. Each analysis used a random effects model to determine the standardised mean difference (SMD) (95% confidence interval, significance p < 0.05) in continuous QOL scores from the relevant physical, psychological, or social domain. Within the three categories of the primary analysis, studies were further divided into subgroups according to QOL questionnaire. This was done to elucidate differences in the size and direction of the effect of UBM on QOL assessed using the different tools. Pooled effect sizes were categorised as small (SMD = 0.2), medium (SMD = 0.5), or large (SMD = 0.8) [33]. Studies reporting physical, psychological, and social wellbeing using multiple assessment tools were included once in each analysis for SMD, with preference for including scores from cancer-specific questionnaires.
In the sensitivity analyses, only studies with subjective reporting of UBM were included. This was done to elucidate if the effect of subjectively reported UBM on QOL differed significantly to that observed in the primary analysis (i.e. subjective and/or objective UBM). Sensitivity analysis including studies with objective reporting of UBM could not be completed due to data availability. Funnel plots for each of the primary analyses were generated in Review Manager (v5.4.1) (The Cochrane Collaboration) [32] to assess publication bias. Low publication bias was inferred when studies were evenly distributed either side of the main effect [27, 34].
Exploratory analyses
Exploratory meta-analyses were performed with studies grouped according to the QOL assessment tool employed. These analyses used a random effects model to determine mean difference (MD) (95% confidence interval, significance p < 0.05) between UBM + and UBM − groups in QOL scores within the domains of each questionnaire. The mean difference between groups was compared to the questionnaire’s Minimal Clinically Important Difference (MCID) or Minimal Important Difference (MID), subject to their availability in the literature. The MCID and MID represent the minimum change in QOL score necessary for an individual to perceive an improvement or deterioration in wellbeing. Comparison to MID or MCID was completed to add clinical relevance to the results of the analysis, to improve the translation of findings into practice [27, 35].
Results
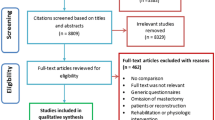
The database search yielded 16,916 records. After duplicates were removed, 11,470 records were entered for title and abstract screening. Seven hundred and twenty-seven records were included for full-text screening from which a further 668 were excluded due to reasons outlined in Fig. 1. Fifty-eight records were included in the systematic review, of which 39 were suitable for inclusion in a meta-analysis. Four studies were reported across multiple publications [15, 24, 36, 37]. Results from the publication with the most complete dataset were included in analysis.
Prisma flow diagram for systematic review process [26]
A summary of studies included in the systematic review can be found in Table 1. Types of UBM reported were lymphoedema (n = 31) of the upper-limb (n = 30) or breast (n = 1); chronic upper-body pain (n = 14), including post-mastectomy pain syndrome (n = 5), breast specific pain (n = 1), and lymphatic pain (n = 1); upper-body disability (n = 1); impaired shoulder ROM (n = 1); or a combination of upper-body symptoms and functional limitations (n = 11) (Table 1).
Fifty-seven studies reported the methods used to determine the presence of UBM, and these were self-report/questionnaire responses (n = 34), objective measures (n = 14), or a combination of the two (n = 9). One study did not describe the method used to categorise participants as lymphoedema positive or negative [38]. Questionnaires used alone or in combination to assess UBM included the McGill Pain Questionnaire [39] (n = 3), Brief Pain Inventory [40] (n = 2), Disabilities of the Arm, Shoulder and Hand questionnaire [41] (n = 2), Visual Analogue Scale [42] (n = 4), lymphoedema and pain questionnaire [43] (n = 1), Douleur Neuropathique-4 questionnaire [44] (n = 1), unspecified/custom UBM/Lymphoedema questionnaire (n = 5), The Breast Cancer and Lymphedema Symptom Experience Index (BCLE-SEI) [45] (n = 1), Functional Assessment of Cancer Therapy, Breast-Arm Symptom Subscale [46] (n = 1), or the “breast swelling” item on the EORTC QLQ-BR23 questionnaire [47] (n = 1). Objective measures used to identify lymphoedema were upper-limb circumference (n = 11), perometry (n = 1), bioelectrical impedance (n = 1), and volumetric displacement (n = 1). Impaired shoulder ROM was quantified using goniometry (n = 3).
QOL was assessed using the following tools: Medical Outcomes Study – Short form 36 (SF-36) [48] (n = 19); European Organisation for Research and Treatment of Cancer, Quality of life Questionnaire – Core (EORTC QLQ-C30) [49] (n = 13) and/or breast module (EORTC QLQ-BR23) [47] (n = 4); Functional Assessment of Cancer Therapy, Breast (FACT-B) [46] (n = 5) with arm symptoms subscale (FACT-B + 4) [50] (n = 9); Medical Outcomes Study – Short form 12 [51] (n = 4); Lymphedema Functioning Disability and health questionnaire for upper-limb lymphedema (LYMPH-ICF-UL) [52] (n = 3); World Health Organisation Quality of Life Questionnaire, brief (WHOQOL-BREF) [53] (n = 2); 20-item Quality of life questionnaire [54] (n = 1); Psychological General Well-Being index (PGWB) [55] (n = 1); The Quality of Life scale – Patient version [56] (n = 1); The Quality of Life scale – Breast Cancer version [57] (n = 1), and the European Quality of Life 5 Dimensions 3 Level Version questionnaire (EQ-5D-3L) [58] (n = 1).
Statistically significant differences between UBM + and UBM − groups existed across several QOL domains. Groups with lymphoedema [14, 38, 59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79], pain [54, 64, 80,81,82,83,84,85,86,87,88], movement limitations [4, 64], upper-body disability [89], or a combination of UBM types [16, 18, 90,91,92,93] reported poorer QOL than UBM − groups in at least one domain. Where QOL was not significantly different between groups [94,95,96], or no statistical analysis was presented [97] mean or median subscale scores tended to be lower in those with UBM compared to those without [94, 95, 98,99,100,101,102], particularly with respect to physical symptoms. Few studies reported trends towards superior QOL in UBM − groups, in terms of severity of arm symptoms [103] and physical wellbeing [18, 96, 99, 101], mental wellbeing [96], and global QOL, physical role, emotional role, cognitive functioning and social functioning [18].
Primary analysis
Physical wellbeing was reported in 28 studies using eight different QOL assessment tools. The relevant physical wellbeing, physical functioning, or physical component scores from eight QOL assessment tools were included in the meta-analysis. Overall, physical wellbeing was significantly poorer in the UBM + group, with UBM exerting a large negative effect on scores in this domain across all questionnaires (SMD = − 0.99; 95%CI = − 1.26, − 0.71; Z = 7.00; df = 27; p < 0.00001) [Total (n = 10,501); UBM + (n = 3334); UBM − (n = 7167)] (Fig. 2).
Psychological/emotional wellbeing was reported in 25 studies using eight QOL assessment tools. Psychological/emotional wellbeing was significantly poorer in the UBM + group with a moderate effect size (SMD = − 0.43; 95%CI = − 0.60, − 0.27; Z = 5.05; df = 24; p < 0.00001) [Total (n = 8225); UBM + (n = 3021); UBM − ( n = 5204)] (Fig. 3). There was evidence to suggest a significant negative effect of UBM for psychological/emotional wellbeing measured using the SF-36 (p < 0.00001), FACT-B (p = 0.001), EORTC-QLQ C30 (p < 0.00001), and ‘other’ questionnaires (p < 0.0001). There was no between group differences in SF-12 questionnaire scores (p = 0.32).
Social wellbeing/function was reported in 28 studies using seven QOL assessment tools. Overall, social wellbeing/function was significantly poorer in the UBM + group, with a moderate to large effect size (SMD = − 0.62; 95%CI = − 0.83, − 0.40; Z = 5.68; df = 27; p < 0.00001) [Total (n = 10,160); UBM + (n = 3355); UBM − ( n = 6805)] (Fig. 4). Moderate and large significant negative effects of UBM were observed in studies using the SF-36 (SMD = − 0.52; 95%CI = − 0.71, − 0.32; Z = 5.19; df = 11; p < 0.00001) and EORTC QLQ-C30 questionnaires, respectively (SMD = − 1.16; 95%CI = − 1.74, − 0.58; Z = 3.92; df = 4; p < 0.00001) and ‘other’ questionnaires (SMD = − 1.30; 95%CI = − 2.62, 0.02; Z = 1.93; df = 2; p < 0.00001). No significant differences were observed between groups for the FACT-B (p = 0.38) or WHOQOL-Bref (p = 0.98) questionnaires.
The sensitivity analysis (Online resource 1) showed that excluding studies which used objective measures of UBM had a minor impact on the magnitude, but not on the direction or significance of the effect of UBM on QOL. Including individuals with objective UBM (e.g. clinically diagnosed lymphoedema) in the analysis does not significantly diminish the size of the effect, irrespective of whether they experience adverse symptoms (e.g. discomfort) or not.
Study quality
The results of the study quality assessment are summarised in Fig. 5 and presented in full in Online resource 1. Results are displayed as the proportion of included studies meeting each JBI checklist item. Of the 58 included studies, 72.4% were rated as good quality. Of those studies included in the meta-analysis, 71.8% were rated as good quality. Reasons for poor quality ratings included insufficient description of the study inclusion criteria and sample characteristics, failure to describe the criteria for the classification into UBM + and UBM − groups, lack of appropriate statistical analysis, and inadequate controlling of confounding variables.
Quality of included studies: Joanna Briggs Institute checklist for analytical cross-sectional studies [30]
Evaluation of publication bias
Funnel plots for each of the primary analyses showed asymmetrical distribution of studies either side of the main effect (Online resource 1) inferring the presence of publication bias, such as failure to publish small studies with insignificant effects estimates. This may have contributed to an overestimation of the effect of UBM on wellbeing scores.
Exploratory analyses
In the exploratory analyses, studies were grouped according to QOL questionnaire. Domain scores were compared between UBM + and UBM − groups. Differences in scores were given clinical context by way of comparison to predetermined MID or MCID thresholds [27, 35], available for some widely used and validated questionnaires including the SF-36, SF-12, and EORTC QLQ-C30 [35, 104, 105]. UBM demonstrated a negative effect of clinically important magnitude, across all subscales of the SF-36 and SF-12 questionnaires. Furthermore, there was a significant negative effect on physical and social health scores on the WHOQOL-BREF questionnaire due to UBM. No difference existed between UBM + and UBM − groups for EORTC QLQ-C30 emotional or cognitive functioning, EORTC QLQ-BR23 body image, sexual function, sexual enjoyment, arm symptoms, or future perspectives, or FACT-B + 4 social/family wellbeing. Findings from the exploratory analysis are summarised in Table 2. Forest plots from each analysis are available in the supplementary material (Online resource 1).
Discussion
The aim of the present study was to evaluate the effect of breast cancer treatment-related UBM on QOL. The primary analyses demonstrated that physical, psychological/emotional, and social aspects of QOL were negatively impacted by the presence of UBM after treatment. However, the degree to which each of these domains was affected, varied. Difference in QOL was most substantial in terms of physical wellbeing and function, as would be expected given the presence of physical upper-body symptoms and limitations differentiating the two groups. Detriment to physical QOL domains has previously been attributed to the difficulty UBM introduces to performing routine tasks such as cooking, cleaning, dressing/grooming and driving [106, 107]. The present analysis also revealed that beyond being a source of physical morbidity, UBM is associated with impairment to social function and psychological wellbeing. This echoes findings from studies that have identified UBM as a source of distress and psychological burden [107]. Experiencing UBM may magnify the discrepancy between one’s pre- and post-cancer capabilities — for example, the inability to perform usual roles within home, social and work context — explaining to some extent, why UBM contributes to impaired psychological and social wellbeing [14, 16, 24, 107, 108].
The review included studies that reported QOL after breast cancer using a variety of general or cancer-specific multidimensional QOL tools, warranting exploratory analyses with studies grouped according to questionnaire. These analyses also revealed substantial impairment across several domains of QOL due to UBM. However, the direction and size of the effect of UBM on corresponding subscales of different questionnaires varied (Table 2), and in some instances, contrasted findings from the primary analysis. For example, UBM had no effect on social functioning or social/family wellbeing subscales of the EORTC QLQ-C30 and FACT-B questionnaires, respectively, yet demonstrated a negative effect on SF-36 social function and WHOQOL-BREF social relationships subscales. Effects were also inconsistent between questionnaires for emotional functioning, general health/global QOL, and breast/arm symptoms subscales. The variable impact of UBM on QOL according to questionnaire may be accounted for by disparities in the number of studies included in each exploratory analysis. Other factors including sample demographics, treatment regime, and UBM type, duration, and severity, have been identified as moderators of the effect of UBM on QOL and may have contributed to the variable effects observed [109,110,111].
It is also worth considering the potential impact of questionnaire selection, on assessing QOL across the cancer continuum [112, 113]. Cancer-specific questionnaires, designed to assess QOL during active treatment when patients experience acute treatment side effects, new psychosocial stressors, and fears about the future, may not contain items of relevance to longer term cancer survivors [114,115,116]. Conversely, generic assessment tools fail to capture the presence of specific cancer/treatment-related effects and their impact on QOL. Selecting a tool with coverage of concerns relevant to a person’s stage on the cancer continuum is paramount to accurate and informative QOL assessment [112]. To improve detection of impaired QOL going forward, administration of a combination of cancer-specific and generic questionnaires may be indicated.
This review represents a comprehensive study of the literature describing multiple types of UBM and their relationship to QOL. It is the first to produce a meta-analysis quantifying the overall effect of UBM on key QOL domains, and the effect of UBM on QOL scores from individual questionnaires.
Study limitations
There are limitations to consider, the first related to the types of UBM reported and methods used to categorise individuals as UBM + or UBM − . The majority of included studies compared individuals with or without lymphoedema. As a prevalent type of UBM after breast cancer there is merit in assessing the impact of lymphoedema on QOL, but findings of these meta-analyses may not reflect the impact of other types of UBM on QOL. Furthermore, the dichotomous classification of UBM represents a limitation to appreciating the complexities of its effect on QOL. For example, the influence of UBM severity, UBM duration/time since treatment, and UBM type is obscured by categorising individuals into discrete UBM + and UBM − groups. A comprehensive meta-analysis in which UBM is further stratified according to type and severity and accounts for time since treatment may address this limitation. However, this may not be feasible given the heterogeneity of currently available data, and the potential co-occurrence of multiple types of UBM (e.g. pain associated with lymphoedema).
Second, as QOL is a multidimensional construct, this review sought to determine the differential impact of UBM on multiple life domains. As such, only studies that employed multidimensional QOL assessment tools were included. Studies using questionnaires to assess components of wellbeing such as anxiety and depression severity, functional impairment, or body image, were excluded. Viewed alongside this review these measures may add richness to the understanding of breast cancer survivor experiences of UBM after treatment.
Finally, the risk of bias and potential overestimation of the observed effect should be addressed. Funnel plots generated for the primary analysis were asymmetrical, inferring risk of publication bias [34]. Additional sources of bias may have included the poor reporting and methodological quality, evident in the ‘poor’ quality rating given to ~ 30% of studies, and the high level of heterogeneity between studies in terms of time since treatment, UBM type, and criteria for assignment to UBM + and UBM − groups existed between studies.
Clinical implications
Whilst this review does not provide evidence endorsing strategies to prevent or manage UBM, the findings justify efforts taken to minimise the presence and impact of UBM to preserve QOL. In the literature to date, examples of such strategies include the selection of minimally invasive procedures to minimise the risk of developing UBM [117,118,119,120,121]; implementation of “Prehabilitation” to improve physical and psychological condition prior to initiating breast cancer treatment and promote superior treatment outcomes [122,123,124,125,126,127]; and the implementation of “Rehabilitation”, such as physical therapy/exercise or activities to promote recovery to pre-treatment physical capacity and QOL [126,127,128,129,130]. Based on the findings of this review, there is merit in implementing UBM prevention and management strategies that address multiple aspects of wellbeing, in order to effectively minimise impairment to overall QOL [7, 131].
Conclusions
Individuals with breast cancer-related UBM that persists beyond primary treatment, report significantly poorer QOL than individuals without UBM. While the most substantial negative effects were observed in physical wellbeing and functioning domains, evidence showed that several domains of QOL are subject to impairment in groups with UBM. There is merit in assessing impairment due to UBM using relevant, multidimensional QOL assessment tools. The pursuit of strategies to prevent and manage UBM is warranted, to minimise its impact on physical, psychological, and social wellbeing across the cancer continuum.
Data availability
Template data collection forms and extracted data used for analysis are available upon reasonable request to the corresponding author.
References
Sung H, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA: Cancer J Clin. 2021;71(3):209–49. https://doi.org/10.3322/caac.21660.
Heilat G, Brennan M, French J. Management of early stage breast cancer. Aust J Gen Pract. 2019;48:604–8. https://doi.org/10.31128/AJGP-03-19-4891.
Hayes SC, et al. Upper-body morbidity after breast cancer: incidence and evidence for evaluation, prevention, and management within a prospective surveillance model of care. Cancer. 2012;118(8 Suppl):2237–49. https://doi.org/10.1002/cncr.27467.
Aerts PDM, et al. The relationship between morbidity after axillary surgery and long-term quality of life in breast cancer patients: the role of anxiety. Eur J Surg Oncol : J Eur Soc Surg Oncol Br Assoc Surg Oncol. 2011;37(4):344–9. https://doi.org/10.1016/j.ejso.2011.01.016.
Lee CH, et al. Effect of breast cancer surgery on chest tightness and upper limb dysfunction. Medicine. 2019;98(19):e15524. https://doi.org/10.1097/MD.0000000000015524.
Fallowfield L, Jenkins V. Psychosocial/survivorship issues in breast cancer: are we doing better? J Natl Cancer Inst. 2015;107(1):335. https://doi.org/10.1093/jnci/dju335.
Loh SY, Musa AN. Methods to improve rehabilitation of patients following breast cancer surgery: a review of systematic reviews. Breast Cancer (Dove Medical Press). 2015;7:81–98. https://doi.org/10.2147/BCTT.S47012.
Jung BF, et al. Neuropathic pain following breast cancer surgery: proposed classification and research update. Pain. 2003;104(1–2):1–13. https://doi.org/10.1016/s0304-3959(03)00241-0.
Shamley D, et al. Changes in shoulder muscle size and activity following treatment for breast cancer. Breast Cancer Res Treat. 2007;106(1):19–27. https://doi.org/10.1007/s10549-006-9466-7.
Stan D, Loprinzi CL, Ruddy KJ. Breast cancer survivorship issues. Hematol Oncol Clin North Am. 2013;27(4):805–ix. https://doi.org/10.1016/j.hoc.2013.05.005.
Mols F, et al. Quality of life among long-term breast cancer survivors: a systematic review. Eur J Cancer. 2005;41(17):2613–9. https://doi.org/10.1016/j.ejca.2005.05.017.
de Ligt KM, et al. The impact of health symptoms on health-related quality of life in early-stage breast cancer survivors. Breast Cancer Res Treat. 2019;178(3):703–11. https://doi.org/10.1007/s10549-019-05433-3.
Hayes S, et al. Upper-body morbidity following breast cancer treatment is common, may persist longer-term and adversely influences quality of life. Health Qual Life Outcomes. 2010;8(1):92. https://doi.org/10.1186/1477-7525-8-92.
Jørgensen MG, et al. The impact of lymphedema on health-related quality of life up to 10 years after breast cancer treatment. npj Breast Cancer. 2021;7(1):70. https://doi.org/10.1038/s41523-021-00276-y.
Nesvold I-L, et al. Arm/shoulder problems in breast cancer survivors are associated with reduced health and poorer physical quality of life. Acta Oncol. 2010;49(3):347–53. https://doi.org/10.3109/02841860903302905.
Nesvold I-L, et al. The relation between arm/shoulder problems and quality of life in breast cancer survivors: a cross-sectional and longitudinal study. J Cancer Survivorship : Res Pract. 2011;5(1):62–72. https://doi.org/10.1007/s11764-010-0156-4.
Kootstra JJ, Dijkstra PU, Rietman H, et al. A longitudinal study of shoulder and arm morbidity in breast cancer survivors 7 years after sentinel lymph node biopsy or axillary lymph node dissection. Breast Cancer Res Treat. 2013;139:125–134. https://doi.org/10.1007/s10549-013-2509-y
Kwan W, et al. Chronic arm morbidity after curative breast cancer treatment: prevalence and impact on quality of life. J Clin Oncol. 2002;20(20):4242–8. https://doi.org/10.1200/JCO.2002.09.018.
Wang L, et al. Predictors of persistent pain after breast cancer surgery: a systematic review and meta-analysis of observational studies. CMAJ : Can Med Assoc J = J Assoc Med Can. 2016;188(14):E352–61. https://doi.org/10.1503/cmaj.151276.
Hernandes JC, et al. Quality of life of women who practice dance: a systematic review protocol. Syst Rev. 2018;1:92. https://doi.org/10.1186/s13643-018-0750-5.
Rietman JS, et al. Late morbidity after treatment of breast cancer in relation to daily activities and quality of life: a systematic review. Eur J Surg Oncol (EJSO). 2003;29(3):229–38. https://doi.org/10.1053/ejso.2002.1403.
Hidding JT, et al. Treatment related impairments in arm and shoulder in patients with breast cancer: a systematic Review. PLoS ONE. 2014;9(5):e96748. https://doi.org/10.1371/journal.pone.0096748.
Pusic AL, et al. Quality of life among breast cancer patients with lymphedema: a systematic review of patient-reported outcome instruments and outcomes. J Cancer Survivorship : Res Pract. 2013;7(1):83–92. https://doi.org/10.1007/s11764-012-0247-5.
Engel J, et al. Predictors of quality of life of breast cancer patients. Acta Oncol. 2003;42(7):710–8. https://doi.org/10.1080/02841860310017658.
Ernst MF, et al. Early and late morbidity associated with axillary levels I-III dissection in breast cancer. J Surg Oncol. 2002;79(3):151–5. https://doi.org/10.1002/jso.10061.
Page MJ, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. https://doi.org/10.1136/bmj.n71.
Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.3 (updated February 2022). Cochrane, 2022. Available from www.training.cochrane.org/handbook.
Ouzzani M, et al. Rayyan—a web and mobile app for systematic reviews. Syst Rev. 2016;5(1):210. https://doi.org/10.1186/s13643-016-0384-4.
Covidence systematic review software, Veritas Health Innovation, Melbourne, Australia. Available at www.covidence.org.
Moola S, Munn Z, Tufanaru C, Aromataris E, Sears K, Sfetcu R, Currie M, Lisy K, Qureshi R, Mattis P, Mu P. Chapter 7: Systematic reviews of etiology and risk. In: Aromataris E, Munn Z (Editors). JBI Manual for Evidence Synthesis. JBI, 2020. Available from https://synthesismanual.jbi.global. https://doi.org/10.46658/JBIMES-20-08
Aromataris E, Munn Z (Editors). JBI Manual for Evidence Synthesis. JBI, 2020. Available from https://synthesismanual.jbi.global. https://doi.org/10.46658/JBIMES-20-01
Review Manager (RevMan) [Computer program]. Version 5.4, The Cochrane Collaboration, 2020.
Cohen J. Statistical power analysis for the behavioral sciences. Academic press; 2013 Sep 3.
Sterne JAC, et al. Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. BMJ. 2011;343:d4002. https://doi.org/10.1136/bmj.d4002.
Jayadevappa R, Cook R, Chhatre S. Minimal important difference to infer changes in health-related quality oflife; a systematic review. J Clin Epidemiol. 2017;89:188–98. https://doi.org/10.1016/j.jclinepi.2017.06.009.
Amichetti M, Caffo O. Pain after quadrantectomy and radiotherapy for early-stage breast cancer: incidence, characteristics and influence on quality of life. Results from a retrospective study. Oncology. 2003;65(1):23–8. https://doi.org/10.1159/000071201.
Bulley C, et al. A Morbidity Screening Tool for identifying fatigue, pain, upper limb dysfunction and lymphedema after breast cancer treatment: a validity study. Eur J Oncol Nurs. 2014;18(2):218–27. https://doi.org/10.1016/j.ejon.2013.10.006.
Sürmeli M, Çinar Özdemir Ö. Examination of the relationship between upper limb function, posture and quality of life in patients with and without lymphedema after breast cancer surgery. Konuralp Tıp Dergisi. 2019;11(3):432–9. https://doi.org/10.18521/ktd.595753.
Melzack R. The McGill Pain Questionnaire: major properties and scoring methods. Pain. 1975;1(3):277–99. https://doi.org/10.1016/0304-3959(75)90044-5.
Daut RL, Cleeland CS, Flanery RC. Development of the Wisconsin Brief Pain Questionnaire to assess pain in cancer and other diseases. Pain. 1983;17(2):197–210. https://doi.org/10.1016/0304-3959(83)90143-4.
Angst F, et al. Measures of adult shoulder function: Disabilities of the Arm, Shoulder, and Hand Questionnaire (DASH) and Its Short Version (QuickDASH), Shoulder Pain and Disability Index (SPADI), American Shoulder and Elbow Surgeons (ASES) Society Standardized Shoulder Assessment Form, Constant (Murley) Score (CS), Simple Shoulder Test (SST), Oxford Shoulder Score (OSS), Shoulder Disability Questionnaire (SDQ), and Western Ontario Shoulder Instability Index (WOSI). Arthritis Care Res. 2011;63(S11):S174–88. https://doi.org/10.1002/acr.20630.
Carlsson AM. Assessment of chronic pain. I. Aspects of the reliability and validity of the visual analogue scale. Pain. 1983;16(1):87–101. https://doi.org/10.1016/0304-3959(83)90088-X.
Paskett ED, Stark N. Lymphedema: Knowledge, Treatment, and Impact Among Breast Cancer Survivors. Breast J. 2000;6(6):373–8. https://doi.org/10.1046/j.1524-4741.2000.99072.x.
Bouhassira D, et al. Comparison of pain syndromes associated with nervous or somatic lesions and development of a new neuropathic pain diagnostic questionnaire (DN4). Pain. 2005;114(1–2):29–36. https://doi.org/10.1016/j.pain.2004.12.010.
Fu MR, et al. Symptom report in detecting breast cancer-related lymphedema. Breast Cancer (Dove Med Press). 2015;7:345–52. https://doi.org/10.2147/bctt.S87854.
Brady MJ, et al. Reliability and validity of the functional assessment of cancer therapy-breast quality-of-life instrument. J Clin Oncol. 1997;15(3):974–86. https://doi.org/10.1200/JCO.1997.15.3.974.
Sprangers MA, et al. The European Organization for Research and Treatment of Cancer breast cancer-specific quality-of-life questionnaire module: first results from a three-country field study. J Clin Oncol. 1996;14(10):2756–68. https://doi.org/10.1200/JCO.1996.14.10.2756.
Ware JE Jr. SF-36 Health Survey. In M. E. Maruish (Ed.), The use of psychological testing for treatment planning and outcomes assessment (pp. 1227–1246). Lawrence Erlbaum Associates Publishers; 1999.
Aaronson NK, et al. The European Organization for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst. 1993;85(5):365–76. https://doi.org/10.1093/jnci/85.5.365.
Coster S, Poole K, Fallowfield LJ. The validation of a quality of life scale to assess the impact of arm morbidity in breast cancer patients post-operatively. Breast Cancer Res Treat. 2001;68(3):273–82. https://doi.org/10.1023/a:1012278023233.
Ware JE, Kosinski M, Bowker D, Gandek B, Ware J, Turner-Bowker D. User’s manual for the SF-12v2 health survey. 2002.
Devoogdt N, et al. Lymphoedema Functioning, Disability and Health questionnaire (Lymph-ICF): reliability and validity. Phys Ther. 2011;91(6):944–57. https://doi.org/10.2522/ptj.20100087.
THE WHOQOL GROUP. Development of the World Health Organization WHOQOL-BREF Quality of Life Assessment. Psychological Medicine. Cambridge University Press; 1998;28(3):551–8.
Caffo O, et al. Pain and quality of life after surgery for breast cancer. Breast Cancer Res Treat. 2003;80(1):39–48. https://doi.org/10.1023/A:1024435101619.
Wenger NK, et al. Assessment of quality of life in clinical trials of cardiovascular therapies. Am J Cardiol. 1984;54(7):908–13. https://doi.org/10.1016/s0002-9149(84)80232-5.
Padilla GV, et al. Quality of life index for patients with cancer. Res Nurs Health. 1983;6(3):117–26. https://doi.org/10.1002/nur.4770060305.
Ferrell BR, et al. Quality of life in breast cancer survivors: implications for developing support services. Oncol Nurs Forum. 1998;25(5):887–95.
Rabin R, d Charro F. 2001 EQ-5D: a measure of health status from the EuroQol Group. Ann Med. 2001;33(5):337–43. https://doi.org/10.3109/07853890109002087.
Ahmed RL, et al. Lymphedema and quality of life in breast cancer survivors: the Iowa Women’s Health Study. J Clin Oncol. 2008;26(35):5689–96.
Beaulac SM, et al. Lymphedema and quality of life in survivors of early-stage breast cancer. Arch Surg. 2002;137(11):1253–7. https://doi.org/10.1001/archsurg.137.11.1253.
Bundred N, et al. Increases in arm volume predict lymphoedema and quality of life deficits after axillary surgery: a prospective cohort study. Br J Cancer. 2020;123(1):17–25. https://doi.org/10.1038/s41416-020-0844-4.
Chachaj A, et al. Physical and psychological impairments of women with upper limb lymphedema following breast cancer treatment. Psychooncology. 2010;19(3):299–305. https://doi.org/10.1002/pon.1573.
Koehler L, et al. Quality of life in breast cancer survivors: An assessment of international breast cancer dragon boat racers. Lymphology. 2020;53(4):195–203.
Hau E, et al. The impact of breast cosmetic and functional outcomes on quality of life: long-term results from the St. George and Wollongong randomized breast boost trial. Breast Cancer Res Treat. 2013;139(1):115–23. https://doi.org/10.1007/s10549-013-2508-z.
Heiney SP, et al. Quality of life and lymphedema following breast cancer. Lymphology. 2007;40(4):177–84.
Hormes JM, et al. Impact of lymphedema and arm symptoms on quality of life in breast cancer survivors. Lymphology. 2010;43(1):1–13.
Lopez Penha TR, et al. The quality of life in long-term breast cancer survivors with breast cancer related lymphedema. Acta Chir Belg. 2014;114(4):239–44.
Mak SS, et al. Lymphedema and quality of life in Chinese women after treatment for breast cancer. Eur J Oncol Nurs. 2009;13(2):110–5. https://doi.org/10.1016/j.ejon.2009.01.005.
Pyszel A, et al. Disability, psychological distress and quality of life in breast cancer survivors with arm lymphedema. Lymphology. 2006;39(4):185–92.
Ridner SH. Quality of life and a symptom cluster associated with breast cancer treatment-related lymphedema. Support Care Cancer. 2005;13(11):904–11. https://doi.org/10.1007/s00520-005-0810-y.
Round T, Hayes SC, Newman B. How do recovery advice and behavioural characteristics influence upper-body function and quality of life among women 6 months after breast cancer diagnosis? Support Care Cancer. 2006;14(1):22–9. https://doi.org/10.1007/s00520-005-0838-z.
Togawa K, et al. Self-reported symptoms of arm lymphedema and health-related quality of life among female breast cancer survivors. Sci Rep. 2021;11(1):10701. https://doi.org/10.1038/s41598-021-89055-0.
Velanovich V, Szymanski W. Quality of life of breast cancer patients with lymphedema. Am J Surg. 1999;177(3):184–7. https://doi.org/10.1016/s0002-9610(99)00008-2. (discussion 188).
Wilson RW, Hutson LM, Vanstry D. Comparison of 2 quality-of-life questionnaires in women treated for breast cancer: the RAND 36-Item Health Survey and the Functional Living Index-Cancer. Phys Ther. 2005;85(9):851–60.
Young-Afat DA, et al. Breast edema following breast-conserving surgery and radiotherapy: patient-reported prevalence, determinants, and effect on health-related quality of life. JNCI Cancer Spectr. 2019;3(2):pkz011. https://doi.org/10.1093/jncics/pkz011.
Yusof K, et al. Cross-Cultural Adaptation of the Functional Assessment of Cancer Therapy-Breast (FACT-B) in Malaysian Breast Cancer Survivors. Asian Pac J Cancer Prev. 2021;22(4):1055–61. https://doi.org/10.31557/APJCP.2021.22.4.1055.
Yusof KM, et al. Assessment of potential risk factors and skin ultrasound presentation associated with breast cancer-related lymphedema in long-term breast cancer survivors. Diagnostics. 2021;11(8):1303. https://doi.org/10.3390/diagnostics11081303.
Zhao H, Wu Y, Tao Y, Zhou C, De Vrieze T, Li X, Chen L. Psychometric Validation of the Chinese Version of the Lymphedema Functioning, Disability, and Health Questionnaire for Upper Limb Lymphedema in Patients With Breast Cancer–Related Lymphedema. Cancer Nursing. 2022;45(1):70–82. https://doi.org/10.1097/NCC.0000000000000848
Neuner JM, et al. Quality of life among a population-based cohort of older patients with breast cancer. Breast. 2014;23(5):609–16. https://doi.org/10.1016/j.breast.2014.06.002.
Bell RJ, et al. Persistent breast pain 5 years after treatment of invasive breast cancer is largely unexplained by factors associated with treatment. J Cancer Surviv. 2014;8(1):1–8. https://doi.org/10.1007/s11764-013-0306-6.
Beyaz SG, et al. Postmastectomy pain: a cross-sectional study of prevalence, pain characteristics, and effects on quality of life. Chin Med J (Engl). 2016;129(1):66–71. https://doi.org/10.4103/0366-6999.172589.
Carpenter JS, et al. Postmastectomy/postlumpectomy pain in breast cancer survivors. J Clin Epidemiol. 1998;51(12):1285–92. https://doi.org/10.1016/s0895-4356(98)00121-8.
Hamood R, et al. Chronic pain and other symptoms among breast cancer survivors: prevalence, predictors, and effects on quality of life. Breast Cancer Res Treat. 2018;167(1):157–69.
Gong Y, et al. Prevalence of postmastectomy pain syndrome and associated risk factors: A large single-institution cohort study. Medicine (Baltimore). 2020;99(20):e19834. https://doi.org/10.1097/MD.0000000000019834.
Kaur N, et al. Postmastectomy chronic pain in breast cancer survivors: an exploratory study on prevalence, characteristics, risk factors, and impact on quality of life. Indian J Surg. 2017;80(6):592–8. https://doi.org/10.1007/s12262-017-1663-6.
Macdonald L, et al. Long-term follow-up of breast cancer survivors with post-mastectomy pain syndrome. Br J Cancer. 2005;92(2):225–30. https://doi.org/10.1038/sj.bjc.6602304.
Meijuan Y, et al. A retrospective study of postmastectomy pain syndrome: incidence, characteristics, risk factors, and influence on quality of life. ScientificWorldJournal. 2013;20:159732. https://doi.org/10.1155/2013/159732.
Recchia TL, Prim AC, Luz CM. Upper Limb Functionality and Quality of Life in Women with Five-Year Survival after Breast Cancer Surgery. Rev Bras Ginecol Obstet. 2017;39(3):115–22. https://doi.org/10.1055/s-0037-1598642.
DiSipio T, et al. What determines the health-related quality of life among regional and rural breast cancer survivors? Aust N Z J Public Health. 2009;33(6):534–9.
Casso D, Buist DS, Taplin S. Quality of life of 5–10 year breast cancer survivors diagnosed between age 40 and 49. Health Qual Life Outcomes. 2004;2:25. https://doi.org/10.1186/1477-7525-2-25.
Engel J, et al. Axilla surgery severely affects quality of life: results of a 5-year prospective study in breast cancer patients. Breast Cancer Res Treat. 2003;79(1):47–57. https://doi.org/10.1023/a:1023330206021.
Jariwala P, Kaur N. A descriptive study on prevalence of arm/shoulder problems and its impact on quality of life in breast cancer survivors. Indian J Cancer. 2021;58(2):201–6. https://doi.org/10.4103/ijc.IJC_22_19.
Kibar S, Dalyan Aras M, Unsal Delialioglu S. The risk factors and prevalence of upper extremity impairments and an analysis of effects of lymphoedema and other impairments on the quality of life of breast cancer patients. Eur J Cancer Care. 2017;26(4):e12433. https://doi.org/10.1111/ecc.12433.
Dawes DJ, et al. Impact of lymphoedema on arm function and health-related quality of life in women following breast cancer surgery. J Rehabil Med. 2008;40(8):651–8. https://doi.org/10.2340/16501977-0232.
Hickey OT, et al. Persistent pain after mastectomy with reconstruction. J Clin Anesth. 2011;23(6):482–8. https://doi.org/10.1016/j.jclinane.2011.01.009.
Mandelblatt JS, et al. Sequelae of axillary lymph node dissection in older women with stage 1 and 2 breast carcinoma. Cancer. 2002;95(12):2445–54. https://doi.org/10.1002/cncr.10983.
Batenburg MCT, et al. Patient-Reported Symptoms of Late Toxicity in Patients With Breast Cancer Treated With Hypofractionated Radiation Therapy and the Association With Quality of Life. Int J Radiat Oncol*Biol*Phys. 2023;115(5):1181–91. https://doi.org/10.1016/j.ijrobp.2022.11.008.
Lee SH, et al. Health-related quality of life in breast cancer patients with lymphedema who survived more than one year after surgery. J Breast Cancer. 2012;15(4):449–53. https://doi.org/10.4048/jbc.2012.15.4.449.
Oliveri JM, et al. Arm/hand swelling and perceived functioning among breast cancer survivors 12 years post-diagnosis: CALGB 79804. J Cancer Surviv. 2008;2(4):233–42. https://doi.org/10.1007/s11764-008-0065-y.
Pinto M, et al. Upper limb function and quality of life in breast cancer related lymphedema: a cross-sectional study. Eur J Phys Rehabil Med. 2013;49(5):665–73.
Speck RM, et al. Changes in the Body Image and Relationship Scale following a one-year strength training trial for breast cancer survivors with or at risk for lymphedema. Breast Cancer Res Treat. 2010;121(2):421–30. https://doi.org/10.1007/s10549-009-0550-7.
Vassard D, et al. Psychological consequences of lymphoedema associated with breast cancer: a prospective cohort study. Eur J Cancer. 2010;46(18):3211–8. https://doi.org/10.1016/j.ejca.2010.07.041.
Popovic-Petrovic S, et al. Secondary lymphedema of the arm, the perception of the disease, self-efficacy and depression as determinants of quality of life in patients with breast cancer. Vojnosanit Pregl. 2018;75(10):961–7. https://doi.org/10.2298/VSP160613006P.
Hays RD, Morales LS. The RAND-36 measure of health-related quality of life. Ann Med. 2001;33(5):350–7. https://doi.org/10.3109/07853890109002089.
Osoba D, et al. Interpreting the significance of changes in health-related quality-of-life scores. J Clin Oncol. 1998;16:139–44. https://doi.org/10.1200/jco.1998.16.1.139.
Silver JK, Baima J, Mayer RS. Impairment-driven cancer rehabilitation: An essential component of quality care and survivorship. CA: Cancer J Clin. 2013;63(5):295–317. https://doi.org/10.3322/caac.21186.
Collins LG, et al. Perceptions of upper-body problems during recovery from breast cancer treatment. Support Care Cancer. 2004;12(2):106–13. https://doi.org/10.1007/s00520-003-0554-5.
Recchia TL, Prim AC, Luz CMD. Upper limb functionality and quality of life in women with five-year survival after breast cancer surgery. Rev Bras Ginecol Obstet : Rev Federacao Bras Sociedades Ginecol Obstet TAG. 2017;39(3):115–22. https://doi.org/10.1055/s-0037-1598642.
Chrischilles EA, et al. Upper extremity disability and quality of life after breast cancer treatment in the Greater Plains Collaborative clinical research network. Breast Cancer Res Treat. 2019;175(3):675–89. https://doi.org/10.1007/s10549-019-05184-1.
Rietman JS, et al. Impairments, disabilities and health related quality of life after treatment for breast cancer: a follow-up study 2.7 years after surgery. Disabil Rehabil. 2004;26(2):78–84. https://doi.org/10.1080/09638280310001629642.
Boquiren V, et al. A longitudinal analysis of chronic arm morbidity following breast cancer surgery. Breast Cancer Res Treat. 2016;157(3):413–25. https://doi.org/10.1007/s10549-016-3834-8.
Ballinger RS, Fallowfield LJ. Quality of life and patient-reported outcomes in the older breast cancer patient. Clin Oncol (R Coll Radiol). 2009;21(2):140-155. https://doi.org/10.1016/j.clon.2008.11.003
Sanghera S, et al. Challenges in using recommended quality of life measures to assess fluctuating health: a think-aloud study to understand how recall and timing of assessment influence patient responses. Patient - Patient-Centered Outcomes Res. 2022;15(4):445–57. https://doi.org/10.1007/s40271-021-00555-7.
Maurer T, et al. Health-related quality of life in a cohort of breast cancer survivors over more than 10 years post-diagnosis and in comparison to a control cohort. Cancers. 2021;13(8):1854. https://doi.org/10.3390/cancers13081854.
Chopra I, Kamal KM. A systematic review of quality of life instruments in long-term breast cancer survivors. Health Qual Life Outcomes. 2012;10:14. https://doi.org/10.1186/1477-7525-10-14.
Gotay CC, Muraoka MY. Quality of life in long-term survivors of adult-onset cancers. JNCI: J Natl Cancer Inst. 1998;90(9):656–67. https://doi.org/10.1093/jnci/90.9.656.
Curigliano G, et al. De-escalating and escalating treatments for early-stage breast cancer: the St. Gallen International Expert Consensus Conference on the Primary Therapy of Early Breast Cancer 2017. Ann Oncol : Off J Eur Soc Med Oncol. 2017;28(8):1700–12. https://doi.org/10.1093/annonc/mdx308.
Wazir U, Mokbel K. De-escalation of breast cancer surgery following neoadjuvant systemic therapy. Eur J Breast Health. 2021;18(1):6–12. https://doi.org/10.4274/ejbh.galenos.2021.2021-5-4.
Waks AG, Winer EP. Breast Cancer Treatment: A Review. JAMA. 2019;321(3):288–300. https://doi.org/10.1001/jama.2018.19323.
Giuliano AE, et al. Effect of axillary dissection vs no axillary dissection on 10-year overall survival among women with invasive breast cancer and sentinel node metastasis: The ACOSOG Z0011 (Alliance) Randomized Clinical Trial. JAMA. 2017;318(10):918–26. https://doi.org/10.1001/jama.2017.11470.
Canavese G, et al. Sentinel lymph node biopsy versus axillary dissection in node-negative early-stage breast cancer: 15-year follow-up update of a randomized clinical trial. Ann Surg Oncol. 2016;23(8):2494–500. https://doi.org/10.1245/s10434-016-5177-4.
Santa Mina D, et al. The Case for Prehabilitation Prior to Breast Cancer Treatment. Pm R. 2017;9(92):S305-s316. https://doi.org/10.1016/j.pmrj.2017.08.402.
Carli F, et al. Surgical prehabilitation in patients with cancer: state-of-the-science and recommendations for future research from a panel of subject matter experts. Phys Med Rehabil Clin N Am. 2017;28(1):49–64. https://doi.org/10.1016/j.pmr.2016.09.002.
Treanor C, Kyaw T, Donnelly M. An international review and meta-analysis of prehabilitation compared to usual care for cancer patients. J Cancer Surviv. 2018;12(1):64–73. https://doi.org/10.1007/s11764-017-0645-9.
Yang A, Sokolof J, Gulati A. The effect of preoperative exercise on upper extremity recovery following breast cancer surgery: a systematic review. Int J Rehabil Res. 2018;41(3):189–96. https://doi.org/10.1097/MRR.0000000000000288.
Cancer Council Victoria and Department of Health Victoria. Optimal care pathway for people with breast cancer. 2nd edn. Melbourne: Cancer Council Victoria; 2021.
Olsson Möller U, et al. A comprehensive approach to rehabilitation interventions following breast cancer treatment - a systematic review of systematic reviews. BMC Cancer. 2019;19(1):472. https://doi.org/10.1186/s12885-019-5648-7.
Ribeiro IL, et al. Effectiveness of early rehabilitation on range of motion, muscle strength and arm function after breast cancer surgery: a systematic review of randomized controlled trials. Clin Rehabil. 2019;33(12):1876–86. https://doi.org/10.1177/0269215519873026.
Spence RR, Heesch KC, Brown WJ. Exercise and cancer rehabilitation: a systematic review. Cancer Treat Rev. 2010;36(2):185–94. https://doi.org/10.1016/j.ctrv.2009.11.003.
Bruce J, et al. Exercise versus usual care after non-reconstructive breast cancer surgery (UK PROSPER): multicentre randomised controlled trial and economic evaluation. BMJ. 2021;375:e066542. https://doi.org/10.1136/bmj-2021-066542.
Sandel LS, et al. Dance and movement program improves quality-of-life measures in breast cancer survivors. Cancer Nurs. 2005;4:301–9. https://doi.org/10.1097/00002820-200507000-00011.
Acknowledgements
The authors wish to acknowledge Dr Gordana Popovic (UNSW StatsCentral) for her contribution to the study, through the provision of statistical analysis support.
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions
Author information
Authors and Affiliations
Contributions
EM: study conception, article screening, data extraction, data analysis/interpretation, manuscript composition.
DS: study conception, manuscript review.
NA: article screening, data extraction, manuscript review.
MH: article screening, manuscript review.
KM: article screening, manuscript review.
RW: study conception, article screening, manuscript review.
BC: study conception, article screening, data extraction, data analysis/interpretation, manuscript review.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval
No ethical approval was required for the conduct of this study. The systematic review was conducted in accordance with the PRISMA 2020 statement [26], and the Cochrane handbook for systematic review and meta-analysis [27]. The study was prospectively registered on PROSPERO (CRD42020203445).
Conflict of interest
The authors declare no competing interests to declare that are relevant to the content of this study. Eliza Macdonald received a tuition fee offset via the Australian Government Research Training Program Scholarship program. No additional funding was received for the conduct of this study.
Disclaimer
The authors wish to note that the submitted manuscript is longer than the average, as outlined in the “Journal of Cancer Survivorship” submission guidelines. This is due to the inclusion of sizeable tables and figures, which demonstrate rigorous adherence to PRISMA guidelines and the scale/completeness of the systematic review and meta-analysis. The authors acknowledge that the “Journal of Cancer Survivorship” values high quality, transparent reporting, and welcome feedback to optimise this review for its readership.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rachel E. Ward and Briana K. Clifford are co-senior authors.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Macdonald, E.R., Amorim, N.M.L., Hagstrom, A.D. et al. Evaluating the effect of upper-body morbidity on quality of life following primary breast cancer treatment: a systematic review and meta-analysis. J Cancer Surviv (2023). https://doi.org/10.1007/s11764-023-01395-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11764-023-01395-0