Abstract
Purpose
The purpose of this study is to develop a European Organisation for Research and Treatment of Cancer Quality of Life Group (EORTC QLG) questionnaire that captures the full range of physical, mental, and social health-related quality of life (HRQOL) issues relevant to disease-free cancer survivors. In this phase III study, we pretested the provisional core questionnaire (QLQ-SURV111) and aimed to identify essential and optional scales.
Methods
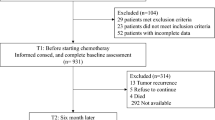
We pretested the QLQ-SURV111 in 492 cancer survivors from 17 countries with one of 11 cancer diagnoses. We applied the EORTC QLG decision rules and employed factor analysis and item response theory (IRT) analysis to assess and, where necessary, modify the hypothesized questionnaire scales. We calculated correlations between the survivorship scales and the QLQ-C30 summary score and carried out a Delphi survey among healthcare professionals, patient representatives, and cancer researchers to distinguish between essential and optional scales.
Results
Fifty-four percent of the sample was male, mean age was 60 years, and, on average, time since completion of treatment was 3.8 years. Eleven items were excluded, resulting in the QLQ-SURV100, with 12 functional and 9 symptom scales, a symptom checklist, 4 single items, and 10 conditional items. The essential survivorship scales consist of 73 items.
Conclusions
The QLQ-SURV100 has been developed to assess comprehensively the HRQOL of disease-free cancer survivors. It includes essential and optional scales and will be validated further in an international phase IV study.
Implications for Cancer Survivors
The availability of this questionnaire will facilitate a standardized and robust assessment of the HRQOL of disease-free cancer survivors.
Similar content being viewed by others
Availability of data and material
The data that support the findings of this study are available from the Division of Psychosocial Research and Epidemiology of the Netherlands Cancer Institute (contact person: L.V. van de Poll-Franse), but restrictions apply to the availability of these data due to an agreement between the Netherlands Cancer Institute and the European Organisation for Research and Treatment of Cancer Quality of Life Group, and so they are not publicly available. Data are available pending approval of both the Netherlands Cancer Institute and the EORTC.
The QLQ-SURV100 can be requested on the website of the EORTC QLG: EORTC Quality of Life Website https://qol.eortc.org/quality-of-life-group/.
Code availability
Not applicable.
Change history
11 March 2022
A Correction to this paper has been published: https://doi.org/10.1007/s11764-022-01199-8
References
Lagergren P, Schandl A, Aaronson NK, Adami HO, de Lorenzo F, Denis L, et al. Cancer survivorship: an integral part of Europe’s research agenda. Mol Oncol. 2019;13(3):624–35. https://doi.org/10.1002/1878-0261.12428.
Ayanian JZ, Jacobsen PB. Enhancing research on cancer survivors. J Clin Oncol. 2006;24(32):5149–53. https://doi.org/10.1200/JCO.2006.06.7207.
Van Leeuwen M, Husson O, Alberti P, Arraras JI, Chinot OL, Costantini A, et al. Understanding the quality of life (QOL) issues in survivors of cancer: towards the development of an EORTC QOL cancer survivorship questionnaire. Health Qual Life Outcomes. 2018;16(1):114. https://doi.org/10.1186/s12955-018-0920-0.
Stewart AL, Ware JE, Jr. Measuring functioning and well-being: the medical outcomes study approach. Durham and London: Duke University Press; 1992.
Kulis D, Bottomley A, Whittaker C, van de Poll-Franse LV, Darlington A, Holzner B, et al. The Use of The Eortc item library to supplement Eortc quality of life instruments. Value Health. 2017;20(9):A775. https://doi.org/10.1016/j.jval.2017.08.2236.
Johnson C, Aaronson N, Blazeby JM, Bottomley A, Fayers P, Koller M, et al. Guidelines for developing questionnaire modules. EORTC Quality of Life Group; 2011.
Keller SD, Bayliss MS, Ware JE Jr, Hsu MA, Damiano AM, Goss TF. Comparison of responses to SF-36 Health Survey questions with one-week and four-week recall periods. Health Serv Res. 1997;32(3):367–84.
Ueda P, Mercer CH, Ghaznavi C, Herbenick D. Trends in frequency of sexual activity and number of sexual partners among adults aged 18 to 44 years in the US, 2000–2018. JAMA Network Open. 2020;3(6):e203833-e. https://doi.org/10.1001/jamanetworkopen.2020.3833.
Karraker A, DeLamater J, Schwartz CR. Sexual frequency decline from midlife to later life. J Gerontol: Ser B. 2011;66B(4):502–12. https://doi.org/10.1093/geronb/gbr058.
Petersen MA, Aaronson NK, Arraras JI, Chie WC, Conroy T, Costantini A, et al. The EORTC CAT Core-The computer adaptive version of the EORTC QLQ-C30 questionnaire. Eur J Cancer. 2018;100:8–16. https://doi.org/10.1016/j.ejca.2018.04.016.
Kuliś D, Bottomley A, Velikova G, Greimel E, Koller M, Group obotEQoL. EORTC quality of life Group translation procedure. EORTC Quality of Life Group; 2017.
Kumthekar P, Raizer J, Singh S. Low-grade glioma. Cancer Treat Res. 2015;163:75–87. https://doi.org/10.1007/978-3-319-12048-5_5.
Brito C, Azevedo A, Esteves S, Marques AR, Martins C, Costa I, et al. Clinical insights gained by refining the 2016 WHO classification of diffuse gliomas with: EGFR amplification, TERT mutations, PTEN deletion and MGMT methylation. BMC Cancer. 2019;19(1):968. https://doi.org/10.1186/s12885-019-6177-0.
Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–83.
NVivo qualitative data analysis Software. 10 ed: QSR International Pty Ltd.; 2012.
Muthén LK, Muthén BO. Mplus. 5.0 ed 2007.
Schermelleh-Engel K, Moosbrugger H, Müller H. Evaluating the fit of structural equation models: Tests of significance and descriptive goodness-of-fit measures. Methods Psychol Res Online. 2003;8(2):23–74.
Bjorner JB, Kosinski M, Ware JE Jr. Calibration of an item pool for assessing the burden of headaches: an application of item response theory to the headache impact test (HIT). Qual Life Res. 2003;12(8):913–33.
Giesinger JM, Kieffer JM, Fayers PM, Groenvold M, Petersen MA, Scott NW, et al. Replication and validation of higher order models demonstrated that a summary score for the EORTC QLQ-C30 is robust. J Clin Epidemiol. 2016;69:79–88. https://doi.org/10.1016/j.jclinepi.2015.08.007.
Hasson F, Keeney S, McKenna H. Research guidelines for the Delphi survey technique. J Adv Nurs. 2000;32(4):1008–15.
Hsu C-C, Sandford BA. The Delphi technique: making sense of consensus. Pract. Assess. Res. Evaluation. 2007;12(1).
Keeney S, Hasson F, McKenna H. Consulting the oracle: ten lessons from using the Delphi technique in nursing research. J Adv Nurs. 2006;53(2):205–12. https://doi.org/10.1111/j.1365-2648.2006.03716.x.
COMET. DelphiManager. COMET; 2016.
Akins RB, Tolson H, Cole BR. Stability of response characteristics of a Delphi panel: application of bootstrap data expansion. BMC Med Res Methodol. 2005;5:37. https://doi.org/10.1186/1471-2288-5-37.
Knowles ES, Byers B. Reliability shifts in measurement reactivity: driven by content engagement or self-engagement? J Pers Soc Psychol. 1996;70(5):1080–90. https://doi.org/10.1037/0022-3514.70.5.1080.
Franke GH. “The whole is more than the sum of its parts”: The effects of grouping and randomizing items on the reliability and validity of questionnaires. Eur J Psychol Assess. 1997;13(2):67–74. https://doi.org/10.1027/1015-5759.13.2.67.
Van de Poll-Franse LV, Mols F, Gundy CM, Creutzberg CL, Nout RA, Verdonck-de Leeuw IM, et al. Normative data for the EORTC QLQ-C30 and EORTC-sexuality items in the general Dutch population. Eur J Cancer. 2011;47(5):667–75.
van Leeuwen M, Kieffer JM, Efficace F, Fosså SD, Bolla M, Collette L, et al. International evaluation of the psychometrics of health-related quality of life questionnaires for use among long-term survivors of testicular and prostate cancer. Health Qual Life Outcomes. 2017;15(1):97. https://doi.org/10.1186/s12955-017-0670-4.
Fayers PM, Hand DJ. Factor analysis, causal indicators and quality of life. Qual Life Res. 1997;6(2):139–50. https://doi.org/10.1023/a:1026490117121.
Fayers PM, Hand DJ. Causal variables, indicator variables and measurement scales: an example from quality of life. 2002;165(2):233-53.https://doi.org/10.1111/1467-985x.02020.
Fayers PM, Hand DJ, Bjordal K, Groenvold M. Causal indicators in quality of life research. Qual Life Res. 1997;6(5):393–406. https://doi.org/10.1023/a:1018491512095.
Kieffer JM, Verrips E, Hoogstraten J. Model specification in oral health-related quality of life research. Eur J Oral Sci. 2009;117(5):481–4. https://doi.org/10.1111/j.1600-0722.2009.00650.x.
Kilburn LS, Banerji J, Bliss JM. The challenges of long-term follow-up data collection in non-commercial, academically-led breast cancer clinical trials: the UK perspective. Trials. 2014;15:379. https://doi.org/10.1186/1745-6215-15-379.
Aaronson NK, Ahmedzai S, Bergman B, Bullinger M, Cull A, Duez NJ, et al. The European organization for research and treatment of cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst. 1993;85(5):365–76.
Cella DF, Tulsky DS, Gray G, Sarafian B, Linn E, Bonomi A, et al. The functional assessment of cancer therapy scale: development and validation of the general measure. J Clin Oncol. 1993;11(3):570–9.
Zhao L, Portier K, Stein K, Baker F, Smith T. Exploratory factor analysis of the cancer problems in living scale: a report from the American cancer society’s studies of cancer survivors. J Pain Symptom Manage. 2009;37(4):676–86.
Zebrack BJ, Ganz PA, Bernaards CA, Petersen L, Abraham L. Assessing the impact of cancer: development of a new instrument for long-term survivors. Psychooncology. 2006;15(5):407–21.
Crespi CM, Ganz PA, Petersen L, Castillo A, Caan B. Refinement and psychometric evaluation of the impact of cancer scale. J Natl Cancer Inst. 2008;100(21):1530–41. https://doi.org/10.1093/jnci/djn340.
Crespi CM, Ganz PA, Petersen L, Smith SK. A procedure for obtaining impact of cancer version 2 scores using version 1 responses. Qual Life Res. 2013;22(1):103–9.
Wyatt G, Kurtz ME, Friedman LL, Given B, Given CW. Preliminary testing of the long-term quality of life (LTQL) instrument for female cancer survivors. J Nurs Meas. 1996;4(2):153–70.
Gordon NH, Siminoff LA. Measuring quality of life of long-term breast cancer survivors: the long term quality of life-breast cancer (LTQOL-BC) Scale. J Psychosoc Oncol. 2010;28(6):589–609. https://doi.org/10.1080/07347332.2010.516806.
Avis NE, Ip E, Foley KL. Evaluation of the quality of life in adult cancer survivors (QLACS) scale for long-term cancer survivors in a sample of breast cancer survivors. Health Qual Life Outcomes. 2006;4:92.
Avis NE, Smith KW, McGraw S, Smith RG, Petronis VM, Carver CS. Assessing quality of life in adult cancer survivors (QLACS). Qual Life Res. 2005;14(4):1007–23.
Ganz PA, Desmond KA, Leedham B, Rowland JH, Meyerowitz BE, Belin TR. Quality of life in long-term, disease-free survivors of breast cancer: a follow-up study. J Natl Cancer Inst. 2002;94(1):39–49.
Alfano CM, McGregor BA, Kuniyuki A, Reeve BB, Bowen DJ, Wilder Smith A, et al. Psychometric evaluation of the brief cancer impact assessment among breast cancer survivors. Oncology. 2006;70(3):190–202. https://doi.org/10.1159/000094320.
Ferrell BR, Dow KH, Grant M. Measurement of the quality of life in cancer survivors. Qual Life Res. 1995;4(6):523–31.
Baker F, Denniston M, Hann D, Gesme D, Reding DJ, Flynn T, et al. Factor structure and concurrent validity of the satisfaction with life domains scale for cancer (SLDS-C). J Psychosoc Oncol. 2007;25(2):1–17.
Bottomley A, Pe M, Sloan J, Basch E, Bonnetain F, Calvert M, et al. Analysing data from patient-reported outcome and quality of life endpoints for cancer clinical trials: a start in setting international standards. Lancet Oncol. 2016;17(11):e510–4. https://doi.org/10.1016/S1470-2045(16)30510-1.
US Food and Drug Administration. Guidance for industry: patient-reported outcome measures: use in medical product development to support labeling claims. Silver Spring, MD. 2009.
European Medicines Agency Committee for Medicinal Products for Human Use. Appendix 2 to the guideline on the evaluation of anticancer medicinal products in man: the use of patient-reported outcome (PRO) measures in oncology studies EMA/CHMP/292464/2014. London, England. 2016.
Bottomley A, Reijneveld JC, Koller M, Flechtner H, Tomaszewski KA, Greimel E. Current state of quality of life and patient-reported outcomes research. Eur J Cancer. 2019;121:55–63. https://doi.org/10.1016/j.ejca.2019.08.016.
Lisy K, Ly L, Kelly H, Clode M, Jefford M. How do we define and measure optimal care for cancer survivors? An online modified reactive Delphi study. Cancers (Basel). 2021;13(10):2299. https://doi.org/10.3390/cancers13102299.
Calvert M, Kyte D, Mercieca-Bebber R, Slade A, Chan AW, King MT, et al. Guidelines for inclusion of patient-reported outcomes in clinical trial protocols: the SPIRIT-PRO extension. JAMA. 2018;319(5):483–94. https://doi.org/10.1001/jama.2017.21903.
Cuzick J. Statistical controversies in clinical research: long-term follow-up of clinical trials in cancer. Ann Oncol. 2015;26(12):2363–6. https://doi.org/10.1093/annonc/mdv392.
Ye M, Du K, Zhou J, Zhou Q, Shou M, Hu B, et al. A meta-analysis of the efficacy of cognitive behavior therapy on quality of life and psychological health of breast cancer survivors and patients. Psychooncology. 2018;27(7):1695–703. https://doi.org/10.1002/pon.4687.
Kim SH, Kim K, Mayer DK. Self-management intervention for adult cancer survivors after treatment: a systematic review and meta-analysis. Oncol Nurs Forum. 2017;44(6):719–28. https://doi.org/10.1188/17.Onf.719-728.
Duncan M, Moschopoulou E, Herrington E, Deane J, Roylance R, Jones L, et al. Review of systematic reviews of non-pharmacological interventions to improve quality of life in cancer survivors. BMJ Open. 2017;7(11): e015860. https://doi.org/10.1136/bmjopen-2017-015860.
Jefford M, Ward AC, Lisy K, Lacey K, Emery JD, Glaser AW, et al. Patient-reported outcomes in cancer survivors: a population-wide cross-sectional study. Support Care Cancer. 2017;25(10):3171–9. https://doi.org/10.1007/s00520-017-3725-5.
Van de Poll-Franse LV, Horevoorts N, Eenbergen MV, Denollet J, Roukema JA, Aaronson NK, et al. The patient reported outcomes following initial treatment and long term evaluation of survivorship registry: scope, rationale and design of an infrastructure for the study of physical and psychosocial outcomes in cancer survivorship cohorts. Eur J Cancer. 2011;47(14):2188–94.
Halpern MT, Argenbright KE. Evaluation of effectiveness of survivorship programmes: how to measure success? Lancet Oncol. 2017;18(1):e51–9. https://doi.org/10.1016/S1470-2045(16)30563-0.
Acknowledgements
We would like to thank all of the cancer survivors for their willingness to participate in this study. We also thank Johannes Giesinger for his statistical advice and Imogen Ramsey for her advice on the Delphi Study.
EORTC survivorship questionnaire development group (in alphabetical order)
Neil K. Aaronson, Division of Psychosocial Research & Epidemiology, The Netherlands Cancer Institute, Amsterdam, The Netherlands
Maria Antonietta Annunziata, Unit of Oncological Psychology, Centro di Riferimento Oncologico di Aviano (CRO) IRCCS, Aviano, Italy
Volker Arndt, Unit of Cancer Survivorship Research, Division of Clinical Epidemiology and Aging Research & Epidemiological Cancer Registry Baden-Württemberg, German Cancer Research Center (DKFZ), Heidelberg, Germany
Juan Ignacio Arraras, Oncology Departments, Complejo Hospitalario de Navarra, Pamplona, Pamplona, Spain
Didier Autran, Pôle Neurosciences Cliniques, Service de Neuro-Oncologie, Aix-Marseille Université, Marseille, France
Hira Bani Hani, King Hussein Cancer Center, Amman, Jordan
Manas Chakrabarti, Columbia Asia Hospital, Kolkata, India
Olivier Chinot, Pôle Neurosciences Cliniques, Service de Neuro-Oncologie, Aix-Marseille Université, Marseille, France
Juhee Cho, Center for Clinical Epidemiology and Cancer Education Center, Samsung Medical Center, School of Medicine Sungkyunkwan University, Seoul, Korea
Rene Aloisio da Costa Vieira,, Postgraduate Program in Oncology, Barretos Cancer Hospital, Barretos, São Paulo, Brazil
Anne-Sophie Darlington, School of Health Sciences, University of Southampton, UK
Philip R. Debruyne, Kortrijk Cancer Centre, General Hospital Groeninge, Kortrijk, Belgium
Linda Dirven, Department of Neurology, Leiden University Medical Center, Leiden, the Netherlands
Daniela Doege, Unit of Cancer Survivorship Research, Division of Clinical Epidemiology and Aging Research & Epidemiological Cancer Registry Baden-Württemberg, German Cancer Research Center (DKFZ), Heidelberg, Germany
Yannick Eller, Centre for Medical Education, University of Dundee (UK)
Martin Eichler, National Center for Tumor Diseases (NCT/UCC), University Hospital Carl Gustav Carus Dresden, Dresden, Germany
Nanna Friðriksdóttir, National University Hospital of Iceland
Ugo De Giorgi, Department of Medical Oncology, IRCCS Istituto Romagnolo per lo Studio dei Tumori (IRST) "Dino Amadori", Meldola 47014, Italy
Ioannis Gioulbasanis, Chemotherapy Department, Larissa General Clinic “E Patsidis”, Athens, Greece
Eva Hammerlid, Department of Otolaryngology Head and Neck Surgery Sahlgrenska University Hospital, Göteborg, Sweden
Mieke van Hemelrijck Translational Oncology & Urology Research (TOUR), School of Cancer and Pharmaceutical Sciences, King’s College London, UK
Silke Hermann, Epidemiological Cancer Registry Baden-Württemberg, German Cancer Research Center (DKFZ), Heidelberg, Germany
Olga Husson Division of Clinical Studies, Institute of Cancer Research, London, UK
Michael Jefford, Department of Health Services Research, Peter MacCallum Cancer Centre, Melbourne, Australia
Christoffer Johansen, Oncology clinic, Finsen Center, Copenhagen
Colin Johnson, University Surgical Unit, University Hospitals Southampton, Southampton, UK
Jacobien M Kieffer, Division of Psychosocial Research & Epidemiology, The Netherlands Cancer Institute, Amsterdam, The Netherlands
Trille Kristina Kjaer, Survivorship and Inequality in Cancer, Danish Cancer Society Research Center, Copenhagen, Denmark
Meropi Kontogianni, Department of Nutrition & Dietetics, School of Health Sciences and Education, Harokopio University, Athens, Greece
Pernilla Lagergren,Department of Molecular medicine and Surgery, Karolinska Institutet, Stockholm, Sweden
Marieke van Leeuwen, Division of Psychosocial Research & Epidemiology, The Netherlands Cancer Institute, Amsterdam, the Netherlands
Emma Lidington, The Royal Marsden NHS Foundation Trust, London, UK
Karolina Lisy, Department of Health Services Research, Peter MacCallum Cancer Centre, Melbourne, Australia
Ofir Morag, Oncology Institute, Chaim Sheba Medical Center, Tel-Hashomer, Israel
Andy Nordin, East Kent Gynaecological Oncology Centre, Margate, UK
Amal S.H. Al Omari, King Hussein Cancer Center, Amman, Jordan
Andrea Pace, Neuroncology Unit, National Cancer Institute Regina Elena, Rome, Italy
Silvia De Padova, Psycho-Oncology Unit, IRCCS Istituto Romagnolo per lo Studio dei Tumori (IRST) “Dino Amadori” 47014 Meldola, Italy
Duska Petranoviæ, Hematology Department, University Clinical Hospital Center Rijeka, Medical faculty University of Rijeka, Rijeka, Croatia
Monica Pinto, Rehabilitation Medicine Unit, Department of StrategicStrategic Health Services , Istituto Nazionale Tumori – IRCCS—Fondazione G. Pascale , Naples, Italy
Lonneke V. van de Poll-Franse, Division of Psychosocial Research & Epidemiology, The Netherlands Cancer Institute, Amsterdam, the Netherlands
John Ramage, Department of Gastroenterology and Hepatology, Hampshire Hospitals NHS Foundation Trust, Basingstoke, UK
Elke Rammant, Translational Oncology & Urology Research (TOUR), School of Cancer and Pharmaceutical Sciences, King’s College London, UK
Jaap Reijneveld, Department of Neurology and Brain Tumor Center Amsterdam, Amsterdam UMC, Amsterdam, The Netherlands
Samantha Serpentini, Unit of Psychoncology—Breast Unit, Istituto Oncologico Veneto (IOV)-IRCCS, Padua, Italy
Sam Sodergren, School of Health Sciences, University of Southampton, UK
Vassilios Vassiliou, Department of Radiation Oncology, Bank of Cyprus Oncology Centre, Nicosia, Cyprus
Irma Verdonck-de Leeuw, Department of Otolaryngology / Head & Neck Surgery, Amsterdam UMC, Amsterdam, The Netherlands
Ingvild Vistad, Department of Gynecology and Obstetrics, Sorlandet Hospital Kristiansand, Norway
Teresa Young, Lynda Jackson Macmillan Centre, Mount Vernon Cancer Centre, Northwood, UK
Funding
This study was funded by the a grant from the European Organisation for Research and Treatment of Cancer Quality of Life Group (006/2016).
Author information
Authors and Affiliations
Consortia
Contributions
All authors contributed to the study conception and design. Material preparation performed by Marieke van Leeuwen, Neil Aaronson, Lonneke van de Poll-Franse, and Teresa Young. Data analysis was carried out by Marieke van Leeuwen, Neil Aaronson, Jacobien Kieffer, and Lonneke van de Poll-Franse. All authors, except for Neil Aaronson, Jacobien Kieffer, and Lonneke van de Poll-Franse, participated in data collection. The first draft of the manuscript was written by Marieke van Leeuwen, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee (Institutional Review Board of the Netherlands Cancer Institute; M17QOL) and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Consent to participate
Informed consent was obtained from all individual participants included in the study.
Consent for publication
Not applicable.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
van Leeuwen, M., Kieffer, J.M., Young, T.E. et al. Phase III study of the European Organisation for Research and Treatment of Cancer Quality of Life cancer survivorship core questionnaire. J Cancer Surviv 17, 1111–1130 (2023). https://doi.org/10.1007/s11764-021-01160-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11764-021-01160-1