Abstract
Purpose
The aim of this study is to assess the societal burden of colorectal cancer (CRC) survivorship 2–10 years post-diagnosis in terms of (1) societal costs, and (2) quality of life/utilities, and to analyze associated patient characteristics.
Methods
This is a cross-sectional, bottom-up prevalence-based burden of disease study, conducted from a societal perspective in the Netherlands. In total, 155 CRC survivors were included. Utilities were measured by the EQ-5D-5L, using the Dutch tariffs. A cost questionnaire was developed to obtain cost information. Subgroup analyses were performed, based on patient characteristics and sensitivity analyses.
Results
Of all CRC survivors, 81(54%) reported no problems for mobility, 133(88%) for self-care, 98(65%) for daily activities, 59(39%) for pain/discomfort, and 112(74%) for anxiety/depression on the EQ-5D-5L. The average EQ-5D-5L utility score was 0.82 (SD = 0.2) on a scale from 0 (death) to 1 (perfect health). Significant differences in utility score were found for gender, tumor stage, number of comorbidities, and lifestyle score. The average societal costs per CRC survivor per 6 months were estimated at €971 (min = €0, max = €32,425). Significant differences in costs were found for the number of comorbidities.
Conclusions
This study shows a considerable burden of CRC survivors 2–10 years after diagnosis, in comparison with survivors sooner after diagnosis and with healthy individuals in the Netherlands.
Implications for Cancer Survivors
Long-term care of CRC survivors should focus on improving the societal burden by identifying modifiable factors, as summarized in the WCRF/AICR lifestyle score, including body composition, physical activity, and diet.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Worldwide, the number of colorectal cancer (CRC) survivors is rising, and continuing growth is expected [1,2,3]. In 2020, over 5.25 million individuals worldwide were estimated to live with a diagnosis of colorectal cancer made in the past five years [4]. Incidence and survival are increasing predominantly due to the aging of the population, technological developments, such as population screening and improved treatment options, and changes in lifestyle factors [5]. Most CRC survivors are elderly individuals with a high risk of recurrence and up to 80% suffer from one or more comorbidities [6, 7]. Additionally, the increasing incidence of CRC in younger adults is a newly arising trend [8]. The introduction of more successful treatments has also increased long-term side effects (e.g. fatigue, peripheral neuropathy, gastrointestinal problems, urinary incontinence, and sexual dysfunction) [6, 9]. Therefore, survivors continue to require care long after diagnosis, which puts constraints on survivors, their family, society, and economy [10, 11].
The burden of disease is often estimated on societal costs and quality of life (QoL) [12, 13]. The annual burden of CRC survivors in the USA seems higher than survivors of breast and prostate cancer [14]. Additionally, the socio-economic status of CRC survivors appears to be more variable than that of breast or prostate cancer survivors, implying that costs are substantially different [11]. Most estimates of CRC costs are based on health services costs for managing the disease and societal costs of premature cancer-related mortality [11, 15]. Furthermore, there is increasing evidence that cancer survivors incur considerable cancer-related time and out-of-pocket costs and lifelong time and travel costs [10, 16], warranting the analysis of societal costs.
Because the long-term survival of CRC patients has risen substantially in the last few decades, there is growing interest in this population’s quality of life [17]. CRC survivors show decreases in social, role, emotional, cognitive, and physical functioning [17, 18]. It is suggested that QoL and symptoms might differ considerably between short-term and long-term survivors [19,20,21,22].
Further, little is known about the influence of patient characteristics on the societal burden of CRC survivors [23]. Previous studies found associations between costs and gender, age, tumor stage, comorbidities, tumor subsite, and time since diagnosis [10, 15, 24,25,26]. Lifestyle factors relevant to the risk of cancer (body composition, physical activity, diet) are summarized in a lifestyle score according to the cancer prevention recommendations of the World Cancer Research Fund (WCRF)/American Institute for Cancer Research (AICR) [1]. The association between this lifestyle score and costs/utility scores in CRC survivors is unknown. A higher WCRF/AICR lifestyle score was found to be associated with better physical functioning and less fatigue [1, 27]. Finding associations between the WCRF/AICR lifestyle score and costs or QoL/utilities might introduce new and early intervention methods in clinical practice, due to its modifiable nature.
The Dutch healthcare system consists of mandatory health insurance for Dutch citizens from private insurers, voluntary complementary insurance, and tax-funded, income-dependent long-term care by the government. Since 2006, citizens are able to freely choose private insurers for the mandatory health insurance, thereby introducing market competition [28]. The content of the mandatory health insurance is determined by the Dutch government, whereas insurers and care providers collectively establish prices. Furthermore, insurers offer voluntary complementary insurance, such as additional physical therapy or dentist care. Since 2015, long-term care for patients complying to the legislative conditions as confirmed by the Care Needs Assessment Centre (CIZ) is financed by income-dependent taxes [29].
To our knowledge, there is no information on Dutch societal costs of long-term CRC survivorship. Worldwide, few studies have analyzed CRC costs from a societal perspective and methodological heterogeneity and lacking transparency are present [30, 31]. Therefore, the aim of this study is to assess the burden of CRC survivors 2–10 years post-diagnosis in terms of (1) societal costs, and (2) QoL/utilities, and to analyze associated patient characteristics, including sociodemographic, clinical and lifestyle characteristics.
Methods
This is a cross-sectional, bottom-up, prevalence-based burden of disease study from a societal perspective in the Netherlands. The study is embedded in the cross-sectional part of the “Energy for life after ColoRectal cancer” (EnCoRe) study, which assesses lifestyle and QoL of CRC survivors. The methods of the EnCoRe study have been published and are briefly described below [23].
Setting, participants, and procedure
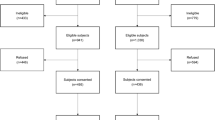
This study consists of patients (> 18 years of age) who have been diagnosed with and treated for stage Ι-ΙΙΙ CRC at Maastricht University Medical Center + (MUMC +) between 2002 and 2010. Patients were identified through the Netherlands Cancer Registry and recruited by mail between May 2012 and December 2013. Exclusion criteria were: (1) stage ΙV disease, (2) passed away, (3) currently no home address in the Netherlands, (4) unable to comprehend the Dutch language, and (5) presence of comorbidities that could obstruct participation. In total, 155 individuals participated in this study. The EnCoRe study was approved by the Medical Ethics Committee of MUMC + and Maastricht University, the Netherlands, and written informed consent was obtained from all participants. Participants underwent several measurements at one point during a house visit by a trained research assistant. Measurements included a general questionnaire, which was developed based on existing questionnaires by the EnCoRe research team and field experts. Additionally, the questionnaire contained questions on medical care, with recall periods of 3/6 months, depending on the estimated frequency of attendance. If estimated attendance for an activity was high, for instance visiting the general practitioner (GP), a recall period of 3 months was chosen to increase reliability. Participants wrote down all medication and supplements used in the past 6 months. The supplement packaging was checked by the research assistant.
Measurements
The main outcome measurements are societal costs (in 2014 Euros) and QoL/utilities. A cost questionnaire was developed by field experts, based on the steps mentioned by Thorn, and pilot tested [32]. QoL was assessed with the European Quality of Life-5 Dimensions-5 Levels (EQ-5D-5L), which includes five domains (mobility, self-care, usual activities, pain-discomfort, and anxiety/depression). Each domain consists of 5 options/levels, ranging from 1 to 5 [33]. The reliability and validity of the EQ-5D-5L in cancer patients has been shown [34,35,36]. The EQ-5D-5L generates a five-dimension health state, which was transformed into a single utility score based on Versteegh et al. [33, 37]. The Dutch tariff showed a single utility score ranging from − 0.446 (worse-than-dead) to 0 (death) to 1 (perfect health) [36,37,38].
Cost analysis and valuation
Costs of individual survivors were calculated for the six months preceding the measurement and were summed (bottom-up approach). Costs were divided into healthcare sector costs, patient and family costs, and costs in other sectors. Healthcare sector costs and patient and family costs were valuated according to the most recently updated Dutch Manual for Cost Analysis in Health Care Research from 2015 [39]. Since this is the most recent Dutch costing manual and the data were collected between 2012 and 2013, all costs are in 2014 Euros. As recommended by this manual, the medication costs were based on www.medicijnkosten.nl and used the price per dose of the drug. If no start- and/or end-date of medication was noted, it was assumed survivors were taking the medication the full 6 months. If the frequency was missing, the lowest entered number by other survivors was assumed (0.5 unit). In case of missing data, the lowest price of the medication was assumed (e.g. lowest dose and cheapest brand). A standard price for supplements was estimated by calculating an average price per supplement from all house-brand supplements offered online by a Dutch store [40]. Medication and supplement prices were transformed from 2016 Euros into estimated 2014 Euros (decrease of 0.2% according to the Dutch Central Bureau of Statistics) [41]. Informal care prices were based on shadow prices (€14/h in 2014) [42]. Travel expenses and productivity losses were calculated according to the updated Dutch manual [39]. Travel expenses were estimated based on the mean distance from a house to a care organization, in kilometers multiplied by the standard cost price per kilometer (€0.19). The friction cost method was used for productivity losses, which multiplies the days of production lost till replacement (85 days) by the average day-wage (€34.75). Conservative estimates (lowest cost price) were used in case of uncertainty.
Statistical methods
Survivors were excluded from the analyses if > 1 item on the EQ-5D-5L was missing. In case of one missing value for the EQ-5D-5L, the population mean was imputed. When medical care questions were missing, the lowest entered population number, excluding zero, was imputed. However, if the total population entered a zero, a zero was imputed. The statistical analyses were performed with SPSS version 25. Seven subgroup analyses for costs and utilities were performed, namely for: gender (male/female), age (< 70/ ≥ 70 years), tumor stage (Stage I/II/III), comorbidities (0/1/ ≥ 2), tumor subsite (colon/rectosigmoid/rectum), WCRF/AICR lifestyle score (low/medium/high; based on tertiles) [1], and time since diagnosis (< 5/ ≥ 5 years). Utility score differences between subgroups were tested for significance by the Mann–Whitney U test, since the data were not normally distributed. Cost differences were tested by non-parametric bootstrapping, simulating 1000 bootstraps to estimate the total cost difference. This method is recommended in literature for analyzing skewed cost data by analyzing arithmetic means and avoiding specific distributional assumptions [43, 44]. The critical p-value was set at 0.05.
Economic evaluations are accompanied by uncertainty. In order for policy makers to correctly interpret the findings it is essential that the uncertainty of point estimates is explored [45]. Three types of sensitivity analyses were performed [46]: (1) using the UK value set to derive utility scores from the EQ-5D-5L and comparing this to the utility scores derived from the Dutch value set, (2) comparing the outcomes of all cases versus all complete cases (no missing data), and (3) removing total cost outliers (≥ 3 SD) from the analyses.
Results
Data were collected from 155 colorectal cancer survivors. Four survivors were excluded, because of > 1 missing item on the EQ-5D-5L. The majority of the resulting 151 participants were male (62.3%), with a mean age of 70 years (SD = 8.7), and mean time since diagnosis of 5.7 years (SD = 1.8). The distribution of tumor stage was: 27.8% Stage Ι, 34.4% Stage ΙΙ, and 32.5% Stage ΙΙΙ. Just over half of survivors presented with 2 or more comorbid conditions (50.3%). Of all participants, 53.0% had a history of colon cancer, 4.6% had a rectosigmoid tumor, and 42.4% had rectal cancer (Table 1).
Quality of life and utility scores
Survivors showed, on a scale from 1 to 5 on the EQ-5D-5L subscales, mean values of 1.9 for mobility (SD = 1.0), 1.2 for self-care (SD = 0.7), 1.6 for daily activities (SD = 0.9), 1.9 for pain/discomfort (SD = 0.9), and 1.3 for anxiety/depression (SD = 0.6). The average EQ-5D-5L utility score was 0.8 (SD = 0.2) (Table 2). Males had a significantly higher utility score (0.85; SD = 0.2) than females (0.77; SD = 0.2). Furthermore, stage ΙΙΙ survivors had a significantly higher utility score (0.84; SD = 0.2) than stage Ι survivors (0.78; SD = 0.2). Survivors with two or more comorbidities had significantly lower utility scores (0.75; SD = 0.2) than survivors having one (0.88; SD = 0.1) or zero (0.92; SD = 0.09) comorbidities. Survivors with a low WCRF/AICR lifestyle score had a significantly lower utility score (0.78; SD = 0.2) than those with a medium score (0.82; SD = 0.2), or a high score (0.86; SD = 0.1). No significant differences in utility score for age, tumor subsite, or time since diagnosis were found (Table 3).
Resource use and societal costs
The resource use categories that showed the highest absolute number of users were medication (77.5%), travel costs (81.5%), and medical specialist (64.9%). The largest resource use per average patient was for paramedical care, with a mean of 2.9 (SD = 8.3). The estimated average societal costs per CRC survivor per 6 months were €971 (min = €0, max = €32,425). The highest costs per average survivor were observed for the categories nursing home (€204), medication (€193), and medical specialist (€141). Survivors with a time since diagnosis of ≥ 5 years showed higher total societal costs (€1007) compared to survivors with a time since diagnosis < 5 years (€888). The largest differences in costs between these two groups were for hospital and nursing home costs. Overall, the healthcare sector costs contained the largest mean costs per average patient (€849), followed by patient and family costs (€120), and then costs in other sectors (€2) (Table 4; Fig. 1).
Subgroup costs
Survivors with two or more comorbidities presented with significantly higher costs (€1514) than survivors having one (€528) or zero (€316) comorbidities. There were no significant differences in costs for sex, age, tumor stage, tumor subsite, and WCRF/AICR lifestyle score (Table 5).
Sensitivity analyses
Sensitivity analyses using the UK value set by Devlin et al. (2018) yielded a mean utility score of 0.85 (SD = 0.2; min = 0.1; max = 1.0), compared to the Dutch value set, which resulted in a utility score of 0.82 (SD = 0.2; min = − 0.1; max = 1.0) [47]. Removing incomplete cases (n = 14), resulted in utility scores of 0.83 (SD = 0.2) (Dutch value set) and 0.86 (SD = 0.2) (UK value set), and total societal costs of €987 (SD = 2893.5). After removal of outliers (n = 1), total societal costs were €761 (SD = 994.6; min = 0.0; max = 5679.1). These analyses suggest limited influence of these variations on the outcomes, thus adding to the robustness of this study.
Discussion
The male gender, a higher tumor stage, a lower number of comorbidities, and a higher WCRF/AICR lifestyle score were associated with higher average utility scores. The average societal costs per 6 months were €971, ranging from €0 to €32,425. Significant differences in costs were observed for having ≥ 2 comorbidities compared to one or zero.
The societal costs for CRC survivors are lower compared to the average annual health expenses of the general Dutch population in 2017 (€5100), but are higher compared to the average annual health expenses of individuals with cancer in 2017 (€343) or individuals with CRC (€35) [48, 49]. Additionally, CRC survivors 2–10 years post-diagnosis showed slightly lower utility scores compared to the general Dutch population (0.87) [37], lower utility scores than patients prior to CRC surgery in the Netherlands (0.88) [50], and higher utility scores compared to CRC patients in the primary treatment phase [50].
The highly variable costs are in accordance with previous studies, where the majority of cancer survivors had little or no costs and a small number incurred very high costs [51]. The mean costs in this population were hypothesized to be lower than costs in the primary treatment phase, which several studies have demonstrated to be associated with highest costs [50, 52]. Interestingly, the mean costs of this study were only slightly lower than the costs of rehabilitation (€2106, 6–18 months from diagnosis) and remission (€2812, > 18 months from diagnosis) phase as demonstrated by Färkkilä et al. [52]. This suggests that the societal costs of long-term survivors do not substantially decrease over the years. Additionally, in accordance with Färkkilä et al., survivors ≥ 5 years post-diagnosis demonstrate higher societal costs than survivors < 5 years post-diagnosis [52]. The higher spending by long-term survivors might be explained by their comorbidity burden [53]. A number of studies have suggested an association between the number of comorbidities and costs [14, 54, 55], however, others suggest this association is limited [15, 56]. This study adds to the evidence suggesting an association between comorbidities and costs. The fact that a substantial percentage of survivors in this population presented with two or more comorbidities (50.3%) and the observation that this subgroup showed considerably higher costs and a higher standard deviation than those with zero or one comorbidities suggests that the presence of comorbidities may explain the highly variable costs in the total population.
The QoL in this population appeared to be relatively high compared to the general population [37]. It is well-described in literature that long-term CRC survivors are able to achieve similar QoL scores compared to the general population [57,58,59,60]. Plausible explanations for the high QoL in this population are posttraumatic emotional growth of survivors and positive changes due to the recovery of a possibly fatal condition. Therefore, the comparison of the QoL of cancer survivors with healthy individuals is difficult because of the potential response shift (lowered expectations and a decrease in capabilities might adjust standards) [61]. Quality of life of CRC survivors might also be impacted by improved coping mechanisms and altruism due to positive adaptation, by evaluating personal experiences and goals [62]. The significantly higher utility score in males (0.85) compared to females (0.77) is consistent with the results of Versteegh et al. [37]. Pattamatta et al. state that males oftentimes score their health better in comparison with females [50]. It is suggested that this gender difference in quality of life is explained by lower income, lower educational level, increased household responsibilities, and increased comorbidities of females compared to males [63,64,65]. Additionally, survivors of a stage III tumor showed a significantly higher utility score than survivors of a stage I tumor. A review of the association between tumor stage and QoL demonstrated inconclusive results [66]. The same review also showed strong evidence for comorbidities as a predictor for QoL [66], which is in line with the results of the current study. It should be noted that comparison of utility score studies is complicated by different tariffs that are used for the EQ-5D-5L. This is the first study to have explored the association between the WCRF/AICR lifestyle score and costs/utilities, suggesting that lifestyle, as a modifiable factor, might be of great value in the long-term care of CRC survivors. Additionally, it raises the question whether survivors who improve their WCRF/AICR lifestyle score might thereby improve their utility score.
Strengths of this study are the inclusion of all societal costs, including healthcare sector costs, patient and family costs, and costs in other sectors. Also, missing data is limited. Limitations of the study are, first, measurements were performed at one point in time due to the cross-sectional design. Due to this design, no causal relationship can be established, and quality of life and costs cannot be studied over time [1]. Second, collected data were mostly self-reported and were therefore prone to under- or over-reporting. However, Noben et al. reported that self-reported data can present an adequate estimate of healthcare use [67]. Third, the recall period of 3/6 months can lead to recall bias. Finally, cost calculations were performed based on the Dutch healthcare system and transferability of costs to different healthcare systems should be considered with caution.
In conclusion, this study demonstrates the considerable societal burden of CRC survivors in the Netherlands long after diagnosis. Interestingly, an association between the WCRF/AICR lifestyle score and utility score was found, implying a possible role for lifestyle factors in relation to the burden of CRC survivors. Future studies should focus on replicating these findings in a longitudinal design and study the association between lifestyle scores and cost/utilities. Additionally, future studies should further explore variables, such as particular comorbidities or medical oncologic therapy, that might explain cost/utility differences.
References
Breedveld-Peters JJL, Koole JL, Muller-Schulte E, et al. Colorectal cancers survivors’ adherence to lifestyle recommendations and cross-sectional associations with health-related quality of life. Br J Nutr. 2018;120(2):188–97.
El-Shami K, Oeffinger KC, Erb NL, et al. American Cancer Society Colorectal Cancer Survivorship Care Guidelines. CA Cancer J Clin. 2015;65(6):428–55.
Lucente P. Primary care for survivors of colorectal cancer. JAAPA. 2018;31(12):20–5.
Ferlay J, Lam F, Colombet M, Mery L, Piñeros M, Znaor A, Soerjomataram I, Bray F. Global Cancer Observatory: Cancer Today. International Agency for Research on Cancer; 2020. Available from: https://gco.iarc.fr/today, accessed 26 June 2021.
Torre LA, Siegel RL, Ward EM, et al. Global cancer incidence and mortality rates and trends—An Update. Cancer Epidemiol Biomarkers Prev. 2016;25(1):16–27.
Soerjomataram I, Thong MSY, Ezzati M, et al. Most colorectal cancer survivors live a large proportion of their remaining life in good health. Cancer Causes Control. 2012;23(9):1421–8.
Denlinger CS, Engstrom PF. Colorectal cancer survivorship: movement matters. Cancer Prev Res. 2011;4(4):502–11.
Keum N, Giovannucci E. Global burden of colorectal cancer: emerging trends, risk factors and prevention strategies. Nat Rev Gastroenterol Hepatol. 2019;16(12):713–32.
Boyette-Davis JA, Eng C, Wang XS, et al. Subclinical peripheral neuropathy is a common finding in colorectal cancer patients prior to chemotherapy. Clin Cancer Res. 2012;18(11):3180–7.
Hanly P, Ceilleachair AO, Skally M, et al. How much does it cost to care for survivors of colorectal cancer? Caregiver’s time, travel and out-of-pocket costs. Support Care Cancer. 2013;21(9):2583–92.
Céilleachair AÓ, Hanly P, Skally M, et al. Counting the cost of cancer: out-of-pocket payments made by colorectal cancer survivors. Support Care Cancer. 2017;25(9):2733–41.
Hall PS, Hamilton P, Hulme CT, et al. Costs of cancer care for use in economic evaluation: a UK analysis of patient-level routine health system data. Br J Cancer. 2015;112(5):948–56.
Jo C. Cost-of-illness studies: concepts, scopes, and methods. Clin Mol Hepatol. 2014;20(4):327–37.
Zheng Z, Yabroff KR, Guy GP, Jr., et al. Annual medical expenditure and productivity loss among colorectal, female breast, and prostate cancer survivors in the United States. J Natl Cancer Inst 2016;108(5):djv382.
Mariotto AB, Warren JL, Zeruto C, et al. Cancer-attributable medical costs for colorectal cancer patients by phases of care: what is the effect of a prior cancer history? J Natl Cancer Inst Monogr. 2020;2020(55):22–30.
Yabroff KR, Warren JL, Knopf K, et al. Estimating patient time costs associated with colorectal cancer care. Med Care. 2005;43(7):640–8.
Jansen L, Koch L, Brenner H, et al. Quality of life among long-term (⩾5 years) colorectal cancer survivors – Systematic review. Eur J Cancer. 2010;46(16):2879–88.
Arndt V, Koch-Gallenkamp L, Jansen L, et al. Quality of life in long-term and very long-term cancer survivors versus population controls in Germany. Acta Oncol. 2017. https://doi.org/10.1080/0284186x.2016.1266089:1-8.
Eyl RE, Xie K, Koch-Gallenkamp L, et al. Quality of life and physical activity in long-term (≥5 years post-diagnosis) colorectal cancer survivors - systematic review. Health Qual Life Outcomes. 2018;16(1):112.
Jansen L, Herrmann A, Stegmaier C, et al. Health-related quality of life during the 10 years after diagnosis of colorectal cancer: a population-based study. J Clin Oncol. 2011;29(24):3263–9.
Jansen L, Koch L, Brenner H, et al. Quality of life among long-term (>/=5 years) colorectal cancer survivors—systematic review. Eur J Cancer. 2010;46(16):2879–88.
Denlinger CS, Barsevick AM. The challenges of colorectal cancer survivorship. J Natl Compr Canc Netw. 2009;7(8):883–93 (quiz 894).
van Roekel EH, Bours MJ, de Brouwer CP, et al. The applicability of the international classification of functioning, disability, and health to study lifestyle and quality of life of colorectal cancer survivors. Cancer Epidemiol Biomarkers Prev. 2014;23(7):1394–405.
Adams SV, Ceballos R, Newcomb PA. Quality of life and mortality of long-term colorectal cancer survivors in the Seattle Colorectal Cancer Family Registry. PLoS One. 2016;11(6):e0156534.
Wodchis WP, Arthurs E, Khan AI, et al. Cost trajectories for cancer patients. Curr Oncol. 2016;23(Suppl 1):S64-75.
Fortner BV, Demarco G, Irving G, et al. Description and predictors of direct and indirect costs of pain reported by cancer patients. J Pain Symptom Manage. 2003;25(1):9–18.
van Veen MR, Mols F, Bours MJL, et al. Adherence to the World Cancer Research Fund/American Institute for Cancer Research recommendations for cancer prevention is associated with better health-related quality of life among long-term colorectal cancer survivors: results of the PROFILES registry. Support Care Cancer. 2019;27(12):4565–74.
Mikkers M. The Dutch Healthcare System in International Perspective. Tilburg University, School of Economics and Management; 2016. https://research.tilburguniversity.edu/en/publications/the-dutch-healthcare-system-in-internationalperspective. Accessed 15/02/2021.
Ministerie van Volksgezondheid WeS. Het Nederlandse zorgstelsel. 2016. https://www.rijksoverheid.nl/documenten/brochures/2016/02/09/het-nederlandse-zorgstelsel. Accessed 15/02/2021.
Lorgelly PK, Neri M. Survivorship burden for individuals, households and society: estimates and methodology. J Cancer Policy. 2018;15:113–7.
Céilleachair AÓ, Hanly P, Skally M, et al. Cost comparisons and methodological heterogeneity in cost-of-illness studies: the example of colorectal cancer. Med Care. 2013;51(4):339–50.
Thorn JC, Coast J, Cohen D, et al. Resource-use measurement based on patient recall: issues and challenges for economic evaluation. Appl Health Econ Health Policy. 2013;11(3):155–61.
Huang W, Yang J, Liu Y, et al. Assessing health-related quality of life of patients with colorectal cancer using EQ-5D-5L: a cross-sectional study in Heilongjiang of China. BMJ Open. 2018;8(12):e022711.
Pickard AS, De Leon MC, Kohlmann T, et al. Psychometric comparison of the standard EQ-5D to a 5 level version in cancer patients. Med Care. 2007;45(3):259–63.
Kim SH, Kim HJ, Lee SI, et al. Comparing the psychometric properties of the EQ-5D-3L and EQ-5D-5L in cancer patients in Korea. Qual Life Res. 2012;21(6):1065–73.
Lee CF, Luo N, Ng R, et al. Comparison of the measurement properties between a short and generic instrument, the 5-level EuroQoL Group’s 5-dimension (EQ-5D-5L) questionnaire, and a longer and disease-specific instrument, the Functional Assessment of Cancer Therapy-Breast (FACT-B), in Asian breast cancer patients. Qual Life Res. 2013;22(7):1745–51.
Versteegh MM, Vermeulen KM, Evers SMAA, et al. Dutch Tariff for the Five-Level Version of EQ-5D. Value Health. 2016;19(4):343–52.
Djalalov S, Rabeneck L, Tomlinson G, et al. A review and meta-analysis of colorectal cancer utilities. Med Decis Making. 2014;34(6):809–18.
Hakkaart-van Roijen L, van der Linden N, Bouwmans C, et al. Kostenhandleiding: Methodologie van kostenonderzoek en referentieprijzen voor economische evaluaties in de gezondheidszorg. https://www.zorginstituutnederland.nl/. Published 2016.
Kruidvat. Vitaminen and Voedingssupplementen. https://www.kruidvat.nl/vitaminen-supplementen/c/20039. Accessed 14/05/2017.
CBS. Prijzen toen en nu. https://www.cbs.nl/nl-nl/visualisatie/2016/11/prijzen-toen-en-nu. Accessed 24/08/2017.
CAK. Uurtarieven. https://www.hetcak.nl/portalserver/portals/cak-portal/pages/k1-2-9-4-uurtarieven.html. Accessed 30/04/2015.
Barber JA, Thompson SG. Analysis of cost data in randomized trials: an application of the non-parametric bootstrap. Stat Med. 2000;19(23):3219–36.
Ng ES-W, Grieve R, Carpenter JR. Two-stage nonparametric bootstrap sampling with shrinkage correction for clustered data. Stata J. 2013;13(1):141–64.
Briggs A. Economics notes: handling uncertainty in economic evaluation. BMJ. 1999;319(7202):120.
Thabane L, Mbuagbaw L, Zhang S, et al. A tutorial on sensitivity analyses in clinical trials: the what, why, when and how. BMC Med Res Methodol. 2013;13:92.
Devlin NJ, Shah KK, Feng Y, et al. Valuing health-related quality of life: an EQ-5D-5L value set for England. Health Econ. 2018;27(1):7–22.
Volksgezondheidenzorg.info. Statline: Kosten van ziekten 2017. https://statline.rivm.nl/#/RIVM/nl/dataset/50050NED/table?ts=15998592214412017. Accessed 15/02/2021.
Volksgezondheidenzorg.info. Kosten van ziekten. https://www.volksgezondheidenzorg.info/onderwerp/kosten-vanziekten/infographic. Accessed 15/02/2021.
Pattamatta M, Smeets BJJ, Evers S, et al. Quality of life and costs of patients prior to colorectal surgery. Expert Rev Pharmacoecon Outcomes Res. 2020;20(2):193–8.
Marti J, Hall PS, Hamilton P, et al. The economic burden of cancer in the UK: a study of survivors treated with curative intent. Psychooncology. 2016;25(1):77–83.
Färkkilä N, Torvinen S, Sintonen H, et al. Costs of colorectal cancer in different states of the disease. Acta Oncol. 2015;54(4):454–62.
Sullivan J, Thornton Snider J, van Eijndhoven E, et al. The well-being of long-term cancer survivors. Am J Manag Care. 2018;24(4):188–95.
Yoon S-J, Kim E-J, Seo H-J, et al. The association between Charlson Comorbidity Index and the medical care cost of cancer: a retrospective study. BioMed Res Int. 2015;2015:259341.
Ding R, Zhu D, He P, et al. Comorbidity in lung cancer patients and its association with medical service cost and treatment choice in China. BMC Cancer. 2020;20(1):250.
Chen AB, Li L, Cronin AM, et al. Estimating costs of care attributable to cancer: does the choice of comparison group matter? Health Serv Res. 2018;53 Suppl 1(Suppl Suppl 1):3227–44.
Ratjen I, Schafmayer C, Enderle J, et al. Health-related quality of life in long-term survivors of colorectal cancer and its association with all-cause mortality: a German cohort study. BMC Cancer. 2018;18(1):1156.
Ramsey SD, Berry K, Moinpour C, et al. Quality of life in long term survivors of colorectal cancer. Am J Gastroenterol. 2002;97(5):1228–34.
Arndt V, Merx H, Stegmaier C, et al. Restrictions in quality of life in colorectal cancer patients over three years after diagnosis: a population based study. Eur J Cancer. 2006;42(12):1848–57.
Caravati-Jouvenceaux A, Launoy G, Klein D, et al. Health-related quality of life among long-term survivors of colorectal cancer: a population-based study. Oncologist. 2011;16(11):1626–36.
Gotay CC, Farley JH, Kawamoto CT, et al. Adaptation and quality of life among long-term cervical cancer survivors in the military health care system. Mil Med. 2008;173(10):1035–41.
Lee KE, Lim KH. Mediation effect of adaptation on the quality of life in patients with gastric cancer undergoing gastrectomy: a structure equation model. Asian Nurs Res. 2019;13(1):38–46.
Cherepanov D, Palta M, Fryback DG, et al. Gender differences in health-related quality-of-life are partly explained by sociodemographic and socioeconomic variation between adult men and women in the US: evidence from four US nationally representative data sets. Qual Life Res. 2010;19(8):1115–24.
Hajian-Tilaki K, Heidari B, Hajian-Tilaki A. Are gender differences in health-related quality of life attributable to sociodemographic characteristics and chronic disease conditions in elderly people? Int J Prev Med. 2017;8:95–95.
Lee KH, Xu H, Wu B. Gender differences in quality of life among community-dwelling older adults in low- and middle-income countries: results from the Study on global AGEing and adult health (SAGE). BMC Public Health. 2020;20(1):114.
Bours MJ, van der Linden BW, Winkels RM, et al. Candidate predictors of health-related quality of life of colorectal cancer survivors: a systematic review. Oncologist. 2016;21(4):433–52.
Noben CY, de Rijk A, Nijhuis F, et al. The exchangeability of self-reports and administrative health care resource use measurements: assessement of the methodological reporting quality. J Clin Epidemiol. 2016;74:93-106.e2.
Acknowledgements
We would like to thank all participants of the EnCoRe study and the health professionals at Maastricht University Medical Center+ involved in the recruitment of participants. We would also like to thank the MEMIC center for data and information management for facilitating the logistic processes and data management of our study. Finally, we would like to thank the research dieticians and research assistants who were responsible for patient inclusion and follow-up, performing home visits, as well as data collection and processing.
Funding
The EnCoRe study was supported by grants from Stichting Alpe d’HuZes within the research program ‘Leven met kanker’ of KWF Kankerbestrijding (the Dutch Cancer Society) (Grant No. UM 2010–4867 and UM 2012–5653); from ERA-NET on Translational Cancer Research (TRANSCAN/Dutch Cancer Society, The Netherlands, project no. UM 2014–6877); and from Kankeronderzoekfonds Limburg as part of Health Foundation Limburg (Grant No. 00005739). The funders had no role in the design of the study, the collection, analysis, and interpretation of the data, the writing of the manuscript, or the decision to submit the manuscript for publication.
Author information
Authors and Affiliations
Contributions
MJLB and MPW were involved in the study design; EHvR, MJLB and MPW performed the study; FECMM, EHvR, MPW and SMAAE analyzed the data; FECMM, EHvR, MJLB, MPW and SMAAE were involved in writing the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. The EnCoRe study was approved by the Medical Ethics Committee of MUMC + and Maastricht University, the Netherlands. Written informed consent was obtained from all individual participants included in the study.
Conflict of interest
Van Roekel was financially supported by Wereld Kanker Onderzoek Fonds (WKOF) as part of the World Cancer Research Fund International grant program (grant no. 2016/1620). Mulder, Bours, Weijenberg, and Evers received no sponsored funding.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Mulder, F.E.C.M., van Roekel, E.H., Bours, M.J.L. et al. The burden of colorectal cancer survivors in the Netherlands: costs, utilities, and associated patient characteristics. J Cancer Surviv 16, 1055–1064 (2022). https://doi.org/10.1007/s11764-021-01096-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11764-021-01096-6