Abstract
Purpose
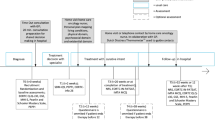
The increasing population of breast cancer survivors highlights the need to (re)consider how we utilize available services for survivorship care in oncology clinics. Electronic Patient-Reported Outcomes (ePROs) can be used to identify patients’ individual care needs and triage them to the right services. We examined the impact on service use, workflow and workload following the introduction of an ePRO-based individual follow-up (PIFU) for women treated for early breast cancer.
Methods
A multi-method approach was used. In a pilot randomized controlled trial, the use of consultations, telephone calls, and specialist referrals were systematically recorded. Comparison was done between PIFU and standard follow-up care (SFU). Focus group interviews with nurse navigators evaluated the impact on workflow and workload qualitatively.
Results
The 64 women randomized to attend SFU used a mean of 3.8 (95% CI: 3.5–4.1) planned consultations during the 2-year study period compared with a mean of 1.9 consultations (95% CI: 1.4–2.4) for the 60 women randomized to PIFU (P < 0.001). Urgent appointments were more frequent in SFU (mean of 0.47 vs 0.22 per patient, P = 0.03). No statistically significant differences were observed in the use of telephone calls and specialist referrals. The nurse navigators did not experience an increase in their workload, but implementation of PIFU may require a re-structured workflow.
Conclusions
The ePRO-based individual follow-up could change organization of care and re-allocate services for those in need of it.
Implications for Cancer Survivors
ePRO-based individual follow-up could potentially ensure more time for those most in need of face-to-face care.



Similar content being viewed by others
References
Senkus E, Kyriakides S, Ohno S, Penault-Llorca F, Poortmans P, Rutgers E, et al. Primary breast cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2015;26:v8–v30.
Allemani C, Minicozzi P, Berrino F, Bastiaannet E, Gavin A, Galceran J, et al. Predictions of survival up to 10 years after diagnosis for European women with breast cancer in 2000-2002. Int J Cancer. 2013;132:2404–12.
Ellegaard MBB, Grau C, Zachariae R, Jensen AB. Women with breast cancer report substantially more disease- and treatment-related side or late effects than registered by clinical oncologists: a cross-sectional study of a standard follow-up program in an oncological department. Breast Cancer Res Treat. 2017;164:727–36.
Danish Breast Cancer Cooperative Group (DBCG). Follow-up [Internet]. DBCG Guidel. 2015 [cited 2019 Oct 10]. p. Chapter 9, 6 pages. Available from: http://www.dbcg.dk/PDFFiler/Kap_9_Opfoelgning_og_kontrol-11.12.2015.pdf.
Rutgers EJ. Follow-up care in breast cancer. Expert Rev Anticancer Ther. 2004;4:212–8.
Lin NU, Thomssen C, Cardoso F, Cameron D, Cufer T, Fallowfield L, et al. International guidelines for management of metastatic breast cancer (MBC) from the European School of Oncology (ESO)-MBC Task Force: surveillance, staging, and evaluation of patients with early-stage and metastatic breast cancer. Breast. 2013;22:203–10.
Johansen C, Dalton SO. Survivorship in new harbors. Acta Oncol (Madr). 2017;56:119–22.
Johansen C, Dalton SO. Survivorship - searching for new directions. Acta Oncol. 2015;54:569–73.
Dalton SO, Johansen C. New paradigms in planning cancer rehabilitation and survivorship. Acta Oncol (Madr). 2013;52:191–4.
Danish Health and Medicines Authority. Follow-up care, Breast cancer [Internet]. 2018 [cited 2019 Oct 10]. Available from: https://www.sst.dk/da/udgivelser/2018/~/media/1C04F012BDEF4F14AED632C457FD0CF2.ashx.
Nelson EC, Eftimovska E, Lind C, et al. Patient reported outcome measures in practice. BMJ. 2015;350:g7818.
Field J, Holmes MM, Newell D. PROMs data: can it be used to make decisions for individual patients? A narrative review. Patient Relat Outcome Meas. 2019;10:233–41.
Basch E, Snyder C. Overcoming barriers to integrating patient-reported outcomes in clinical practice and electronic health records. Ann Oncol. 2017;28:2332–3.
Basch E, Barbera L, Kerrigan CL, et al. Implementation of patient-reported outcomes in routine medical care. Am Soc Clin Oncol Educ B. 2018;38:122–34.
Warrington L, Absolom K, Velikova G. Integrated care pathways for cancer survivors - a role for patient-reported outcome measures and health informatics. Acta Oncol (Madr). 2015;54:600–8.
Bjelic-Radisic V, Dorfer M, Tamussino K, Greimel E. Patients’ view of routine follow-up after breast cancer treatment. Wien Klin Wochenschr. 2017;129:810–5.
Lipscomb J, Gotay CC, Snyder CF. Patient-reported outcomes in cancer: a review of recent research and policy initiatives. CA Cancer J Clin. 2007;57:278–300.
Riis CL, Bechmann T, Jensen PT, Coulter A, Steffensen KD. Are patient-reported outcomes useful in post- treatment follow-up care for women with early breast cancer ? A scoping review. Patient Relat Outcome Meas. 2019;10:117–27.
van Egdom LSE, Oemrawsingh A, Verweij LM, Lingsma HF, Koppert LB, Verhoef C, et al. Implementing patient-reported outcome measures in clinical breast cancer care: a systematic review. Value Health. 2019;22:1197–226.
Halkier B. Focus groups. 1st ed. Samfundslitteratur & Roskilde Universitetsforlag; 2002.
Wong LP. Focus group discussion: a tool for health and medical research. Singap Med J. 2008;49:256–61.
Coleman R, Gray R, Powles T, et al. Adjuvant bisphosphonate treatment in early breast cancer: meta-analyses of individual patient data from randomised trials. Lancet. 2015;386:1353–61.
Bradley R, Burrett J, Clarke M, et al. Aromatase inhibitors versus tamoxifen in early breast cancer: patient-level meta-analysis of the randomised trials. Lancet. 2015;386:1341–52.
Danish Cancer Society. Patient support [Internet]. [cited 2019 Nov 8]. Available from: https://www.cancer.dk/international/patient-support/.
Aaronson NK, Ahmedzai S, Bergman B, et al. The European Organisation for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst. 1993;85:365–76.
European Organisation for Research and Treatment of Cancer. EORTC quality of life questionnaires [Internet]. [cited 2019 Oct 10]. Available from: https://qol.eortc.org/.
Aaronson NK, te Velde A, Hopwood P, et al. The European Organization for Research and Treatment of Cancer breast cancer-specific quality-of-life questionnaire module: first results from a three-country field study. J Clin Oncol. 2017;14:2756–68.
by Ramboll. SurveyXact [Internet]. [cited 2019 Nov 8]. Available from: https://www.surveyxact.com/about-us/.
Riis CL, Jensen PT, Bechmann T, Möller S, Coulter A, Steffensen KD. Satisfaction with care and adherence to treatment when using patient reported outcomes to individualize follow-up care for women with early breast cancer – a pilot randomized controlled trial. Acta Oncol. 2020;59:444–52.
Steine S, Finset A, Laerum E. A new, brief questionnaire (PEQ) developed in primary health care for measuring patients’ experience of interaction, emotion and consultation outcome. Fam Pract. 2001;18:410–8.
Elwyn G, Barr PJ, Grande SW, Thompson R, Walsh T, Ozanne EM. Developing CollaboRATE: a fast and frugal patient-reported measure of shared decision making in clinical encounters. Patient Educ Couns. 2013;93:102–7.
DEFACTUM - Part of Corporate Quality in Central Denmark Region. Indicator objectives for “patient involvement” - theoretical and methodological considerations [Internet]. CFK - Folk. og Kvalitetsudvikling. 2015 [cited 2019 Oct 10]. p. 138. Available from: https://www.defactum.dk/publikationer/ShowPublication?publicationId=519&pageId=309986.
Kvale S, Brinkmann S. Interview (Det kvalitative forskningsinterview som håndværk). 3rd ed. Hans Reitzels Forlag; 2014.
Rodkjær LØ, Bregnballe V, Ågård AS, Handberg CLK. Patient-reported outcomes - a means of facilitating patient involvement. Sygeplejersken. 2015;12:77–80.
Selby P, Velikova G. Taking patient reported outcomes centre stage in cancer research – why has it taken so long? Res Involv Engagem. 2018;4:1–5.
Velikova G, Booth L, Smith AB, Brown PM, Lynch P, Brown JM, et al. Measuring quality of life in routine oncology practice improves communication and patient well-being: a randomized controlled trial. J Clin Oncol. 2004;22:714–24.
Coulter A. Patient engagement—what works? J Ambul Care Manag. 2012;35:80–9.
Kearney N, MacGillivray S, Harrow A, et al. What is the value of the routine use of patient-reported outcome measures toward improvement of patient outcomes, processes of care, and health service outcomes in cancer care? A systematic review of controlled trials. J Clin Oncol. 2014;32:1480–501.
Chen J, Ou L, Hollis SJ. A systematic review of the impact of routine collection of patient reported outcome measures on patients, providers and health organisations in an oncologic setting. BMC Health Serv Res. 2013;13:1–24.
Greenhalgh J, Gooding K, Gibbons E, Dalkin S, Wright J, Valderas J, et al. How do patient reported outcome measures (PROMs) support clinician-patient communication and patient care? A realist synthesis. J Patient-Reported Outcomes. 2018;2:42.
Jensen KP, Back-Pettersson S, Segesten K. “Catching my wavelength”: perceptions of the excellent nurse. Nurs Sci Q. 1996;9:115–20.
Radwin L. Oncology patients’ perceptions of quality nursing care. Res Nurs Health. 2000;23:179–90.
Schougaard LMV, Larsen LP, Jessen A, Sidenius P, Dorflinger L, de Thurah A, et al. AmbuFlex: tele-patient-reported outcomes (telePRO) as the basis for follow-up in chronic and malignant diseases. Qual Life Res. 2016;25:525–34.
Baeksted C, Pappot H, Nissen A, Hjollund NH, Mitchell SA, Basch E, et al. Feasibility and acceptability of electronic symptom surveillance with clinician feedback using the Patient-Reported Outcomes version of Common Terminology Criteria for Adverse Events (PRO-CTCAE) in Danish prostate cancer patients. J Patient-Reported Outcomes. 2017;1:1–11.
Hjollund NHI, Larsen LP, Biering K, et al. Use of patient-reported outcome (PRO) measures at group and patient levels: experiences from the generic integrated PRO system WestChronic. J Med Internet Res. 2014;3:e5.
Howell D, Molloy S, Wilkinson K, Green E, Orchard K, Wang K, et al. Patient-reported outcomes in routine cancer clinical practice: a scoping review of use, impact on health outcomes, and implementation factors. Ann Oncol. 2015;26:1846–58.
Philpot LM, Barnes SA, Brown RM, Austin JA, James CS, Stanford RH, et al. Barriers and benefits to the use of patient-reported outcome measures in routine clinical care: a qualitative study. Am J Med Qual. 2018;33:359–64.
Malterud K. Qualitative research: standards, challenges, and guidelines. Lancet. 2001;358:483–8.
Ministry of Health. Act on research ethics review of health research projects [Internet]. 2011 [cited 2019 Nov 1]. Available from: http://en.nvk.dk/rules-and-guidelines/act-on-research-ethics-review-of-health-research-projects.
Funding
This study was financially supported by the Region of Southern Denmark and by the Danish foundation Trygfonden, Denmark, under Grant ID: 127172.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Ethical approval
The protocol was approved by the Danish Data Protection Agency (2008-58-035) and registered at ClinicalTrials.gov (ID: NCT02935920). According to the Act on Research Ethics Review of Health Research Projects, no approval for this type of study was needed from the Danish Health Research Ethics Committee [49].
Informed consent
Informed consent was obtained from all individual participants included in the study, including the nurse navigators.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 24 kb)
Rights and permissions
About this article
Cite this article
Riis, C.L., Stie, M., Bechmann, T. et al. ePRO-based individual follow-up care for women treated for early breast cancer: impact on service use and workflows. J Cancer Surviv 15, 485–496 (2021). https://doi.org/10.1007/s11764-020-00942-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11764-020-00942-3