Abstract
Purpose
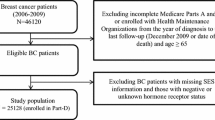
Hormone receptor-positive (HR+) cancers account for most breast cancer diagnoses and deaths. Among survivors with HR + breast cancers, endocrine therapy (ET) reduces 5-year risk of recurrence by up to 40 %. Observational studies in Medicare- and privately-insured survivors suggest underutilization of ET. We sought to characterize ET use in a low-income Medicaid-insured population in North Carolina.
Methods
Medicaid claims data were matched to state cancer registry records for survivors aging 18–64 diagnosed with stage 0–II HR + breast cancer from 2003 to 2007, eligible for ET, and enrolled in Medicaid for at least 12 of 15 months post-diagnosis. We used multivariable logistic regression to model receipt of any ET medication during 15 months post-diagnosis controlling for age, race, tumor characteristics, receipt of other treatments, comorbidity, residence, reason for Medicaid eligibility, involvement in the Breast and Cervical Cancer Control Program (BCCCP), and diagnosis year.
Results
Of 222 women meeting the inclusion criteria, only 50 % filled a prescription for ET. Involvement in the BCCCP and earlier year of diagnoses were associated with significantly higher odds of initiating guideline-recommended ET (adjusted odds ratio [AOR] for the BCCCP 3.76, 95 % confidence interval [CI] 1.67–8.48; AOR for 2004 relative to 2007 2.80, 95 % CI 1.03–7.62; AOR for 2005 relative to 2007 2.11, 95 % CI 0.92–4.85).
Conclusions
Results suggest substantial underutilization of ET in this population. Interventions are needed to improve timely receipt of ET and to better support survivors taking ET.
Implications for Cancer Survivors
Low-income survivors should be counseled on the importance of ET and offered support services to promote initiation and long-term adherence.

Similar content being viewed by others
References
Siegel R, DeSantis C, Virgo K, Stein K, Mariotto A, Smith T, et al. Cancer treatment and survivorship statistics, 2012. CA Cancer J Clin. 2012;62(4):220–41. doi:10.3322/caac.21149.
Burg MA, Grant K, Hatch R. Caring for patients with cancer histories in community-based primary care settings: a survey of primary care physicians in the southeastern US. Prim Health Care Res Dev. 2005;6(03):244–50. doi:10.1191/1463423605pc250oa.
O’Brien KM, Cole SR, Tse CK, Perou CM, Carey LA, Foulkes WD, et al. Intrinsic breast tumor subtypes, race, and long-term survival in the Carolina Breast Cancer Study. Clin Cancer Res. 2010;16(24):6100–10. doi:10.1158/1078-0432.CCR-10-1533.
Early Breast Cancer Trialists’ Collaborative Group (EBCTCG). Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet. 2005;365(9472):1687–717. doi:10.1016/S0140-6736(05)66544-0
Neugut AI, Subar M, Wilde ET, Stratton S, Brouse CH, Hillyer GC, et al. Association between prescription co-payment amount and compliance with adjuvant hormonal therapy in women with early-stage breast cancer. J Clin Oncol. 2011;29(18):2534–42. doi:10.1200/JCO.2010.33.3179.
Hershman DL, Kushi LH, Shao T, Buono D, Kershenbaum A, Tsai WY, et al. Early discontinuation and nonadherence to adjuvant hormonal therapy in a cohort of 8,769 early-stage breast cancer patients. J Clin Oncol. 2010;28(27):4120–8. doi:10.1200/JCO.2009.25.9655.
McCowan C, Shearer J, Donnan PT, Dewar JA, Crilly M, Thompson AM, et al. Cohort study examining tamoxifen adherence and its relationship to mortality in women with breast cancer. Br J Cancer. 2008;99(11):1763–8. doi:10.1038/sj.bjc.6604758.
Hershman DL, Shao T, Kushi LH, Buono D, Tsai WY, Fehrenbacher L, et al. Early discontinuation and non-adherence to adjuvant hormonal therapy are associated with increased mortality in women with breast cancer. Breast Cancer Res Treat. 2011;126(2):529–37. doi:10.1007/s10549-010-1132-4.
Hadji P. Improving compliance and persistence to adjuvant tamoxifen and aromatase inhibitor therapy. Crit Rev Oncol Hematol. 2010;73(2):156–66. doi:10.1016/j.critrevonc.2009.02.001.
Demissie S, Silliman RA, Lash TL. Adjuvant tamoxifen: predictors of use, side effects, and discontinuation in older women. J Clin Oncol. 2001;19(2):322–8.
Kimmick G, Anderson R, Camacho F, Bhosle M, Hwang W, Balkrishnan R. Adjuvant hormonal therapy use among insured, low-income women with breast cancer. J Clin Oncol. 2009;27(21):3445–51. doi:10.1200/JCO.2008.19.2419.
Yung RL, Hassett MJ, Chen K, Gesten FC, Roohan PJ, Boscoe FP, et al. Initiation of adjuvant hormone therapy by Medicaid insured women with nonmetastatic breast cancer. J Natl Cancer Inst. 2012;104(14):1102–5. doi:10.1093/jnci/djs273.
Livaudais JC, Hershman DL, Habel L, Kushi L, Gomez SL, Li CI, et al. Racial/ethnic differences in initiation of adjuvant hormonal therapy among women with hormone receptor-positive breast cancer. Breast Cancer Res Treat. 2011;131(2):607–17. doi:10.1007/s10549-011-1762-1.
Pellegrini I, Sarradon-Eck A, Soussan PB, Lacour AC, Largillier R, Tallet A, et al. Women’s perceptions and experience of adjuvant tamoxifen therapy account for their adherence: breast cancer patients’ point of view. Psychooncology. 2010;19(5):472–9. doi:10.1002/pon.1593.
Dent SF, Gaspo R, Kissner M, Pritchard KI. Aromatase inhibitor therapy: toxicities and management strategies in the treatment of postmenopausal women with hormone-sensitive early breast cancer. Breast Cancer Res Treat. 2011;126(2):295–310. doi:10.1007/s10549-011-1351-3.
Partridge AH, LaFountain A, Mayer E, Taylor BS, Winer E, Asnis-Alibozek A. Adherence to initial adjuvant anastrozole therapy among women with early-stage breast cancer. J Clin Oncol. 2008;26(4):556–62. doi:10.1200/jco.2007.11.5451.
Partridge AH, Wang PS, Winer EP, Avorn J. Nonadherence to adjuvant tamoxifen therapy in women with primary breast cancer. J Clin Oncol. 2003;21(4):602–6.
Wheeler SB, Kohler RE, Goyal RK, Lich KH, Lin C-C, Moore A, et al. Is medical home enrollment associated with receipt of guideline-concordant follow-up care among low-income breast cancer survivors? Med Care. 2013;51(6):494–502. doi:10.1097/MLR.0b013e31828d4d0c.
Khatcheressian JL, Wolff AC, Smith TJ, Grunfeld E, Muss HB, Vogel VG, et al. American Society of Clinical Oncology 2006 update of the breast cancer follow-up and management guidelines in the adjuvant setting. J Clin Oncol. 2006;24(31):5091–7. doi:10.1200/JCO.2006.08.8575.
Cluze C, Rey D, Huiart L, BenDiane MK, Bouhnik AD, Berenger C, et al. Adjuvant endocrine therapy with tamoxifen in young women with breast cancer: determinants of interruptions vary over time. Ann Oncol. 2012;23(4):882–90. doi:10.1093/annonc/mdr330.
Garreau JR, Delamelena T, Walts D, Karamlou K, Johnson N. Side effects of aromatase inhibitors versus tamoxifen: the patients’ perspective. Am J Surg. 2006;192(4):496–8. doi:10.1016/j.amjsurg.2006.06.018.
Kahn KL, Schneider EC, Malin JL, Adams JL, Epstein AM. Patient centered experiences in breast cancer: predicting long-term adherence to tamoxifen use. Med Care. 2007;45(5):431–9. doi:10.1097/01.mlr.0000257193.10760.7f.
Coburn N, Fulton J, Pearlman DN, Law C, DiPaolo B, Cady B. Treatment variation by insurance status for breast cancer patients. Breast J. 2008;14(2):128–34. doi:10.1111/j.1524-4741.2007.00542.x.
Chen AY, Halpern MT, Schrag NM, Stewart A, Leitch M, Ward E. Disparities and trends in sentinel lymph node biopsy among early-stage breast cancer patients (1998–2005). J Natl Cancer Inst. 2008;100(7):462–74.
Voti L, Richardson LC, Reis I, Fleming LE, Mackinnon J, Coebergh JW. The effect of race/ethnicity and insurance in the administration of standard therapy for local breast cancer in Florida. Breast Cancer Res Treat. 2006;95(1):89–95.
Bickell NA, LePar F, Wang JJ, Leventhal H. Lost opportunities: physicians’ reasons and disparities in breast cancer treatment. J Clin Oncol. 2007;25(18):2516–21.
Smith SL, Wai ES, Alexander C, Singh-Carlson S. Caring for survivors of breast cancer: perspective of the primary care physician. Curr Oncol. 2011;18(5):e218–26.
Ganz PA. Survivorship: adult cancer survivors. Prim Care. 2009;36(4):721–41. doi:10.1016/j.pop.2009.08.001.
Potosky A, Han PJ, Rowland J, Klabunde C, Smith T, Aziz N, et al. Differences between primary care physicians’ and oncologists’ knowledge, attitudes and practices regarding the care of cancer survivors. J Gen Intern Med. 2011;26(12):1403–10. doi:10.1007/s11606-011-1808-4.
Shalom MM, Hahn EE, Casillas J, Ganz PA. Do Survivorship care plans make a difference? A primary care provider perspective. J Oncol Pract. 2011;7(5):314–8. doi:10.1200/JOP.2010.000208.
Bradley CJ, Given CW, Roberts C. Race, socioeconomic status, and breast cancer treatment and survival. J Natl Cancer Inst. 2002;94(7):490–6. doi:10.1093/jnci/94.7.490.
Cross CK, Harris J, Recht A. Race, socioeconomic status, and breast carcinoma in the U.S: what have we learned from clinical studies. Cancer. 2002;95(9):1988–99.
Acknowledgments
We would like to thank and recognize our expert advisory committee, consisting of Anne Braswell, Jonathan Fischer, Bo Gamble, Eleanor Greene, Linda Kinney, Patrick Maguire, Brenda McCants, Rachel Raab, LaSonia Roberts-Melvin, and Thea Monet. This research was presented as an abstract to the American Society for Clinical Oncology Annual Research Meeting, held in Chicago on June 1–5, 2012.
Funding
This research was funded by a University Cancer Research Fund Health-e-NC pilot grant (Wheeler) and supported by the Integrated Cancer Information and Surveillance System (ICISS), a UNC Lineberger Comprehensive Cancer Center resource. SW’s time was supported by a NIH Mentored Clinical Scientists Comparative Effectiveness Development Award (1-K-12 HS019468-01 (PI: Weinberger)).
Human subjects and animal studies statement
This research on human subjects was approved by the University of North Carolina at Chapel Hill Institutional Review Board. No animal studies were carried out by the authors for this article.
Conflict of interest statement
The authors have no conflicts to disclose.
Author information
Authors and Affiliations
Corresponding author
Additional information
The contents of this manuscript have not been published previously, except as an abstract accepted for the 2012 American Society of Clinical Oncology meeting (Chicago, IL, June 1–5).
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplemental Table 1
National Drug Codes used to Identify Endocrine Therapy Prescriptions (DOCX 14 kb)
Rights and permissions
About this article
Cite this article
Wheeler, S.B., Kohler, R.E., Reeder-Hayes, K.E. et al. Endocrine therapy initiation among Medicaid-insured breast cancer survivors with hormone receptor-positive tumors. J Cancer Surviv 8, 603–610 (2014). https://doi.org/10.1007/s11764-014-0365-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11764-014-0365-3