Abstract
Traditionally, calcareous beech forests were classified and differentiated according to vascular plants. Bryophytes were often omitted or not all substrates were sampled in relevés, and therefore, the role of bryophytes in plant community differentiation remained unclear. In this paper, we studied bryophyte species richness, composition and functional patterns in vegetation units differentiated by vascular plants. We analysed 45 phytosociological relevés from 400 m2 plots in Fagus sylvatica-dominated forests on dolomite bedrock in Central Slovakia. The most frequent among 59 moss and 8 liverwort species was Tortella tortuosa, followed by Hypnum cupressiforme, Brachytheciastrum velutinum and Ctenidium molluscum. Average richness of bryophytes was 8.1 compared to 38.7 species of vascular plants. Seven bryophyte species were significantly linked to a particular vegetation unit. One-way ANOVA showed no noticeable differences in bryophyte species richness between vegetation units. However, it was negatively affected by xericity and positively by cover of rocks on soil surface. CCA revealed that species composition was affected significantly by xericity, cover of bare rocks and cover of tree layer. Mat and turf life forms prevailed, and both long-lived taxa/perennials and short-lived colonists formed the bryophyte layer. Observed bryophyte species grew mostly on rock, living and dead wood, and only minority of them on soil. Therefore, available substrates greatly contributed to the species richness of bryophytes and total plant diversity of the forest community. A complete investigation of substrates is necessary to assess the drivers of bryophyte species distribution and diversity, and their role in classification of calcareous beech forests.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Forest communies belong to vascular plant-dominated vegetation types. However, their understorey consists not only of the herb layer (herbs, ferns, shrubs and tree juveniles), but frequently also of bryophyte layer (mosses, liverworts, hornworts and lichens). Vascular plants are sampled within plots of phytosociological relevés in all syntaxonomical studies of forest communities using Braun-Blaquet approach (Braun-Blanquet 1964). In spite of the fact that all strata of a terrestric community are considered as a single unit (Westhoff and van der Maarel 1978), sampling of bryophytes in forest communies differs especially according to their proportion in overall species composition. Recording of bryophyte layer species is required especially in acidophilous and boreal, montane or peaty coniferous forests, and bryophyte species were used as diagnostic species (Borhidi 2003; Willner and Grabherr 2007; Chytrý 2013). On the other hand, bryophytes were not usually recorded in eutrophic communities such as beech forests of the Carpino-Fagetea (resp. Querco-Fagetea Br.-Bl. et Vlieger 1937) class (Mucina et al. 2016), where the bryophyte layer is often poorly developed. As they absented in majority of relevés, they were not included in the classification analyses in majority of phytosociological studies. Therefore, bryophytes were not used as diagnostic species in community descriptions on a whole-European level (Willner et al. 2017). They have only rarely been used in classification approaches of forest communities dominated by Fagus sylvatica on a regional level along with vascular plants (e.g.; Grabherr et al. 2003; Tzonev et al. 2006; Willner and Grabherr 2007; Swierkosz et al. 2018) or only exceptionally classified independently from vascular plants (Puglisi et al. 2013, 2016; Alegro et al. 2023). However, bryophytes are good additional indicators of habitat conditions, they show associations with different types of forests (Stefańska-Krzaczek et al. 2022; Alegro et al. 2023) and some studies imply that the most accurate classification is obtained when both vascular plants and bryophytes are used simultaneously to distinguish different vegetation types (Hokkanen 2004).
Beech dominated forests represent natural vegetation of temperate Europe from colline to supramontane zone. They form the most widespread forest plant communities in the Slovak Carpathians. Beech communities were studied already since the first half of the 20th century (Klika 1936; Mikyška 1939). Except for acidophilous beech forests of the Luzulo-Fagion alliance (Slezák et al. 2016), phytosociological sampling and classification was focused on vascular plants. Stronger effort to sample bryophytes in mesotrophic and calcicolous beech forest communities was made only by some authors (Klika 1927; Fajmonová 1971, 1972; Fajmonová and Šimeková 1973). Later, bryophytes were studied in more detail only in the last decades (e.g. Kliment et al. 2010; Ujházyová et al. 2013) in collaboration with specialists who were able to exactly identify bryophyte species. However, many regions are still unexplored and information on bryophytes is missing in majority of phytosociological relevés in the national Slovak Vegetation Database (EU-SK-001; https://www.givd.info/databases.xhtml). Moreover, methodological approaches in bryophyte sampling differed among researchers. Exclusive sampling of terrestial bryophytes prevailed in the previous century. Identification of bryophytes was limited by expert knowledge of a particular author without verification of the identification by a bryologist. Methodological heterogeneity and frequent overlooking of at least part of the bryoflora in the forest understorey led to insufficient knowledge of this component of beech forest communities, and consequently, it was frequently omitted from classification analyses and re-introduced into final tables (Ujházyová et al. 2013; Slezák et al. 2016; Hrivnák et al. 2019). Therefore, an effort was made to unify the methodology of bryophyte sampling in Slovakia in the last decades. As in scree forests or acidophilous communities, other functional groups of bryopyhtes also started to be collected in beech forests, in particular wood-inhabiting and epilithic species (Kliment et al. 2010; Kučera 2010; Valachovič et al. 2014).
General site conditions, mainly water and light availability (Smith 1982; Friedel et al. 2006; Rydin 2009; Király et al. 2013; Stefańska-Krzaczek et al. 2022) and their modification by trees (Caners et al. 2013; Jagodziński et al. 2018) are likely to have the most profound impact on bryophyte species composition in forests. However, some studies show that the availability and heterogeneity of different substrates and microsites such as exposed soil (von Oheimb et al. 2007; Márialigeti et al. 2009), rocks, stumps (Cole et al. 2008), rot holes (Fritz and Heilmann-Clausen 2010), species of trees, volume and different stage of decay of dead wood (Frisvoll and Prestø 1997; Rambo and Muir 1998; Mills and MacDonald 2004, 2005; Márialigeti et al. 2009; Táborská et al. 2015) is the crucial determinant of richness and composition of forest bryophytes. Such availability and heterogeneity of substrates is especially important in Central European beech forests, where leaf litter is one of the most inhibiting factors for bryophyte establishment (Schumacher 2000). Within the different available substrate types, temperature, moisture, light or soil conditions can play an important role in conditioning bryophyte abundance, species composition and diversity (Lee and La Roi 1979; Gustafsson and Eriksson 1995; Vitt et al. 1995; Mills and MacDonald 2005; Moora et al. 2007). These conditions can, however, limit the growth of terricolous and epiphytic species in a different way. Likewise, light can have no significant impact on bryophyte species richness (Humphrey et al. 2002; Mills and MacDonald 2004), but can be one of the most relevant explanatory variables for bryophyte cover (Márialigeti et al. 2009). It is also difficult to generalize the effect of overstorey (tree) species on understorey diversity as the results of various studies range widely and are sometimes conflicting and many studies do not take into account factors related to management practices and site characteristics: Understorey and overstorey may both respond in parallel to site type (Thomsen et al. 2005; Barbier et al. 2008).
Calcareous hilly region surrounding the city of Banská Bystrica belongs to one of the less explored areas of forest communities and bryoflora, although it is a floristically very rich region within the Western Carpathians. Several floristic and phytosociological studies were published describing the vegetation of the area (Futák 1943; Jasík 1992; Galvánek 1999; Janišová 2001; Turis 2001; Turisová and Martincová 2001; Uhliarová and Martincová 2004; Martincová and Ondrášek 2010). Large diversity of plant communities was found here, especially in grasslands. However, the forest vegetation and bryoflora of this region was not systematically studied.
Using original field data from the unexplored region in the Western Carpathians, we tried to reveal bryophyte contribution in beech-dominated forest communities. The objectives of this paper were to (i) explore if bryophyte species composition and species richness differ in vegetation units defined by vascular plants; (ii) assess the influence of environmental variables on bryophyte species richness and composition; (iii) characterize the patterns of bryophyte species traits in the studied forest ecosystems in central part of Slovakia.
Materials and methods
Studied region
We focused on the montaneous region built predominantly by dolomites. The study area is depicted in Fig. 1. This calcareous region is split by the valley of the Hron river and surrounded by larger mountain ranges (the Kremnické vrchy Mts, the Veľká Fatra Mts, the Starohorské vrchy Mts, the Nízke Tatry Mts, the Poľana Mts, the Slovenské rudohorie Mts) and the Zvolenská kotlina basin from the south.
Soil type of majority of studied relevés was identified as Rendzic Leptosols in calciphilous communities and Calcaric Cambisols in mesothrophic ones (soil classification follows FAO 1988). Percentage cover of bare rocks on soil surface varied from 0 to 40%. Elevation ranged from 390 m a.s.l. at the foothills of the southwestern part close to the Hron river up to the highest point Sokolie (975 m a.s.l.) near to Kordíky village.
Mean annual air temperature of this region varies from 5 to 7 °C (Šťastný et al. 2002), mean annual precipitation varies between 750 and 1100 mm (Faško and Šťastný 2002).
According to the phytogeographical classification of Slovakia (Futák 1984) our study region is included in the West-Carpathian flora (Carpaticum occidentale).
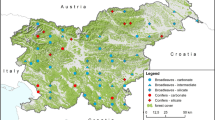
Distribution of relevé plots within the studied area. Colours of symbols correspond to syntaxonomical units (CF – Cephalanthero damasonii-Fagetum sylvaticae subas. typicum; CFc – Cephalanthero damasonii-Fagetum sylvaticae subas. caricetosum albae; ClF – Clematido alpinae-Fagetum sylvaticae; MF – Mercuriali perennis-Fagetum sylvaticae, Dentario enneaphylli-Fagetum sylvaticae subas. caricetosum albae; MFr – Mercuriali perennis-Fraxinetum excelsioris; TF – Teucrio chamaedrys-Fagetum sylvaticae). The map was created using the QGIS program, version 3.28.3 (https://qgis.org/)
Field vegetation sampling and environmental data
Phytosociological relevés containing species and environmental data were collected during the growing seasons in 2011 and 2012. Plot placement followed stratification by following criteria: carbonate (predominantly dolomite) bedrock, beech-dominated stands older than 80 years, canopy closure between 60% and 100%, regular spatial distribution within the area and sampling of visually different plant communities in each locality. A total of 45 phytosociological relevés was made in square or rectangular plots of size 400 m2 where all vascular plants and taxa of terrestrial, epilithic and dead-wood inhabiting bryophytes (E0 layer) were sampled. Epiphytic bryophyte species (growing on living wood such as tree stems, trunks and root swellings) were not recorded - following the standard of phytosociological sampling. Nomenclature of vascular plants follows Marhold and Hindák (1998). Nomenclature and taxonomy of bryophytes follows Hodgetts et al. (2020).
Classification of relevés to the associations of the Braun-Blanquet approach were adopted from the previous study performed on the national level according to vascular plants only (Ujházyová et al. 2021).
A total of seven environmental variables were obtained directly from the sample plots (elevation, slope, cover of bare rocks, cover of herb (E1), shrub (E2) and tree (E3) layers and height of tree layer) to study relationships between bryophyte species composition and diversity and site properties. Xericity index (Austin et al. 1984) was calculated using slope and aspect values measured in field:
Xericity = cos(aspect – 202.5)*tg(slope),
where aspect is the azimuth that a terrain surface faces in degrees and slope is the angle of surface in degrees.
Bryophyte life history traits
We selected nine different traits to analyze the morphology (life form), main life history strategies, as well as geographical (continentality) and ecological attributes (demands for light, temperature, moisture, reaction and nitrogen content – fertility) and substrate preferences (frequency of occurrence on a given substrate) of the recorded bryophytes. We used BRYOATT (Hill et al. 2007) and BryForTrait (Bernhardt-Römermann et al. 2018) datasets of bryophyte species traits, as well as Simmel et al. (2021), Dierßen (2001) and Ellenberg’s indicator values (Ellenberg et al. 1992) to compile trait information for individual species.
Data analysis
Bryophyte species affinity to particular vegetation units was tested by Fisher´s exact test (Chytrý et al. 2002) in the JUICE 7.0 program (Tichý 2002) and their occurrence concentration in the unit was expressed by phi coefficient (Tichý and Chytrý 2006). We used non-parametric Kruskal-Wallis ANOVA to test differences of mean bryophyte and vascular plant species richness among associations in Statistica(r) 12.0 program. To test the relationship between bryophyte species richness and selected environmental variables we used Spearman’s rank correlation coefficient (for elevation, xericity, slope, cover of bare rocks, cover of herb, shrub and tree layers and height of tree layer) and one-way ANOVA (for five vegetation units; Mercuriali perennis-Fraxinetum excelsioris represented only by one relevé was omitted), both in the Statistica 12 (r) software (StatSoft 2013). We used Canonical Correspondence Analysis (CCA) in the software Canoco for Windows 4.5 (Lepš and Šmilauer 2003) to find the most significant environmental variables affecting bryophyte species composition. Their significance was tested using forward selection and Monte Carlo permutation test (999 permutations). Percentage cover values were transformed using logarithmic function to underweight proportion of dominant species in the ordination analysis.
Results
We recorded a total of 67 bryophyte species in 45 phytosociological relevés (compared to 214 vascular plant species). The number of bryophyte species in the forest understorey varied from 0 to 18 in 400 m2 (per relevé), and the average species richness of bryophytes was 8.1, compared to 38.7 species in 400 m2 of vascular plants. The most frequent species in the relevés was Tortella tortuosa (present in 35 relevés), followed by Hypnum cupressiforme (29), Brachytheciastrum velutinum (25), Ctenidium molluscum (25) and Ptychostomum moravicum (20). Out of 67 recorded bryophyte species, 59 were mosses (27 acrocarpous and 32 pleurocarpous) and 8 were liverworts. All species belonged to 11 orders with almost half (47.8%) of them to Hypnales, 19.4% to Dicranales, 8.9% to Bryales and 7.5% to Jungermanniales. The remaining orders had lower percentages but accounted for a total of 16.4%.
Bryophyte species composition and richness variability in vegetation units
Six vegetation units were delimited according to species composition of vascular plants: (TF) Teucrio chamaedrys-Fagetum sylvaticae Ujházyová et Ujházy 2021, (CFt) Cephalanthero damasonii-Fagetum sylvaticae Oberdorfer 1957 subas. typicum, (CFc) Cephalanthero damasonii-Fagetum sylvaticae Oberdorfer 1957 subas. caricetosum albae Ujházyová et Ujházy 2021, (ClF) Clematido alpinae-Fagetum sylvaticae (Sill. 1933) Fajmonová et Uhlířová-Šimeková 1981, (MF) Mercuriali perennis-Fagetum sylvaticae Scamoni 1935, Dentario enneaphylli-Fagetum sylvaticae Oberd. 1957 ex W. Matuszkiewicz et A. Matuszkiewicz 1960 subas. caricetosum albae Ujházyová, Ujházy et Máliš 2013 and (MFr) Mercuriali perennis-Fraxinetum excelsioris (Klika 1942) Husová in Moravec et al. 1982.
Only three bryophyte species were significantly linked to a particular vegetation unit: Homalothecium lutescens to unit TF, Ptychostomum moravicum to CFt and Alleniella besseri to CFc. Another four species (Brachythecium tommasinii, Plagiothecium cavifolium, P. denticulatum and Leskea polycarpa) were bound to scree forest (MFr), but here they were captured by only one relevé. On the other hand, species Ctenidium molluscum and Tortella tortuosa were recorded in all identified units. A full list of recorded species with their percentage frequency and fidelity index in vegetation units is presented in Table 1.
One-way ANOVA showed that there are no noticeable differences in bryophyte species richness between vegetation units (p = 0.787), especially due to a high variance in some of them, in particular in both subassociations of Cephalanthero damasonii-Fagetum sylvaticae (Fig. 2). By contrast, species richness of vascular plants showed highly significant differences among associations (p = 0.003).
Species richness of bryophytes (SR E0 as the number of species per relevé) in vegetation units. Average, standard deviation, minimum and maximum is shown. Differences among averages were not significant (p = 0.787). Abbreviations of vegetation units: CF – Cephalanthero damasonii-Fagetum sylvaticae subas. typicum; CFc – Cephalanthero damasonii-Fagetum sylvaticae subas. caricetosum albae; ClF – Clematido alpinae-Fagetum sylvaticae; MF – Mercuriali perennis-Fagetum sylvaticae, Dentario enneaphylli-Fagetum sylvaticae subas. caricetosum albae; MFr – Mercuriali perennis-Fraxinetum excelsioris; TF – Teucrio chamaedrys-Fagetum sylvaticae
Environmental properties influencing bryophyte species richness and species composition
The results of Spearman’s rank correlation (Table 2; Fig. 3) showed that bryophyte species richness was negatively affected by xericity and positively by cover of bare rocks on soil surface. Cover of bryophytes was positively correlated with bryophyte species richness and also with cover of bare rocks. For comparison, relationships of herb layer with the same variables are shown in the table.
The results of a forward selection performed within CCA analysis showed that species composition is significantly affected only by two environmental variables (xericity and cover of bare rocks) and by percentage cover of tree layer (canopy closure). These three factors accounted for 11.2% of the total variation in species composition (Table 3).
CCA analysis (Fig. 4) revealed that species Exsertotheca crispa, Encalypta streptocarpa, Fissidens dubius, Ctenidium molluscum and partially Homalothecium philippeanum, H. lutescens and Encalypta vulgaris appeared in a positive relation with the cover of bare rocks, while Dicranum montanum, Herzogiella seligeri, Ptychostomum moravicum, Lophocolea heterophylla and Brachytheciastrum velutinum showed a negative relation with the cover of bare rocks. A positive correlation with xericity had species Pseudoleskeella nervosa, Dicranum scoparium, Schistidium apocarpum and Lophocolea heterophylla. Homalothecium philippeanum, Alleniella besseri, Oxyrrhynchium hians, Metzgeria furcata, Pseudanomodon attenuatus and Sciuro-hypnum populeum indicated a strong negative correlation with xericity. Only Schistidium crassipilum ilustrated a stronger positive relation to cover of tree layer and species Encalypta streptocarpa, Homalothecium lutescens and Exsertotheca crispa indicated an accentuated negative relation to tree layer cover.
CCA graph showing a relationship of bryophyte species to the significant environmental variables. Eigenvalues of the 1st axis: 0.28 and the 3rd axis: 0.21. Total inertia in species data: 6.33; Variance explained by selected variables: 0.71. Species names are abbreviated using first four letters of genus and species names (full names are in Table 1)
Trait pattern in the understorey bryophyte layer
Recorded species formed rough mats the most (28.4%), 20.9% of them formed turfs, 16.4% had smooth mats, 14.9% formed tufts, 7.5% had wefts, 6% formed cushions, while the remaining 6% of the species had different life forms (dendroid, fan, thalloid mat or solitary creeping) (Fig. 5a).
More than half (53.7%) of the taxa were perennials (including stress-tolerant and competitive), roughly one third (37.3%) were pioneer colonists or colonists and 9% were long-lived shuttle species (Fig. 5b).
Most taxa were intermediate (56.7%) or subcontinental (26.9%), whereas only a small portion were suboceanic (7.5%), subcontinental/continental (6%) or indifferent (3%) (Fig. 6C). Semi-shade species were the most numerous (70.1%) but a significant portion (20.9%) were semi-light or found in full light, and 6% were shade-loving (Fig. 6L). Two thirds (64.2%) of the taxa were indicators of rather colder climate, 25.4% were indifferent to temperature, while only a smaller portion (10.4%) preferred moderately warm or warm situations (Fig. 6T). Majority of the species (85.1%) preferred moderately moist soils, one species (1,5%) was indifferent to soil moisture and the rest (13.4%) were found on dry soils (Fig. 6M). Two thirds (65.7%) of bryophytes preferred moderately to strongly basic soils but 22.4% were acidophilous (Fig. 6R). Almost two thirds (64.2%) of taxa grew in moderately fertile soils, more than one quarter (28,4%) were indicators of extremely infertile to infertile soils, while only a small portion (4.5%) included species of N-rich substrates and indifferent species (3%) (Fig. 6N).
Average percentage proportion of Ellenberg indicator values (EIV) for continentality (C), light (L), temperature (T), moisture (M), reaction (R) and nutrients (N) of bryophyte species in all 45 relevés. Scale and interpretation of the EIV values is explained in Ellenberg et al. (1992)
Proportionally, species showed habitat or substrate preference (i.e. normally or occasionally found on given substrate) mostly to hard rocks (84.1%), living wood (66.7%), worked rocks (58.7%) or soil (52.4%), but a relatively high preference of bryophytes was also towards soil on rock (46%), decorticated wood (42.9%), gravel or sand (33.3%) and soft rocks (31.7%) (Fig. 7).
Substrate preferences of bryophytes in the study area expressed as the average percentage proportion of bryophyte species in all 45 relevés. RH – rock (hard), RS – rock (soft), RW – rock (worked), SR – soil on rock, SO – soil, PT – peat, GS – gravel or sand, DW – decorticated wood, DV – decaying vegetation, DA – decaying animal, BR – bryophyte, EW – epiphytic on living wood; 3 – a normal habitat or substrate for the species, 2 – an occasional habitat or substrate for the species, 1 – a rare habitat or substrate for the species, 0 – abnormal habitat or substrate for the species
Discussion
Syntaxonomy of bryophytes in beech forests
We proved that species composition of bryophytes in beech forests differed among syntaxonomical units delimited by vascular plants on the dolomites of central Western Carpathians. Number of diagnostic bryophyte species was lower compared to vascular plants. Our results are largely consistent with other studies from the Western Carpathians that at least partly involved bryophytes in the calcareous beech forest classification (Fajmonová 1971, 1972; Fajmonová and Šimeková 1973; Kanka et al. 2008; Kliment et al. 2010; Ujházyová et al. 2013). Similarly to these studies, we found calciphilous (Ctenidium molluscum, Fissidens dubius, Homalothecium lutescens, Pseudoleskeella catenulata, Ptychostomum moravicum, Tortella tortuosa, etc.) along with acidophilous species (Dicranum scoparium, Herzogiella seligeri) and species without a substrate preference (Brachytheciastrum velutinum, Hypnum cupressiforme) in the Cephalanthero-Fagetum and Clematido alpinae-Fagetum associations on the dolomites of Central Slovakia. Among them, Ctenidium molluscum, Dicranum scoparium, Fissidens dubius, Hypnum cupressiforme and Tortella tortuosa can be considered as generalists in calcareous beech forests in the Western Carpathians as they are reported by Fajmonová (1971, 1972), Kanka et al. (2008) and Kliment et al. (2010).
Facultative epiphytic species Ptychostomum moravicum was found diagnostic for Cephalanthero-Fagetum typicum in dolomites of Central Slovakia, whereas species of shaded limestone rocks (Anomodon viticulosus, Leskea polycarpa, Plagiothecium laetum, Porella platyphylla and Pseudanomodon attenuatus) were reported as local differencial species of the same unit in the Muránska planina Mts (Kliment et al. 2010).
The bryophyte layer in montane forests of Clematido alpinae-Fagetum was also rather poor. Along with calciphilous epilithic species, multiple acidophytes of forest understorey were typical for this association in the study area. Above mentioned generalists of calcareous beech forests were constant here. In addition to them, Brachytheciastrum velutinum, Isothecium alopecuroides and Ptychostomum capillare were sampled among the most frequent species in the montane calcareous beech forests in the Veľká Fatra Mts (Kanka et al. 2008; Ujházyová et al. 2013). Differentiation of Clematido alpinae-Fagetum to submontane units on the Central Slovakian dolomites was rather poor, what is partly a consequence of small number of relevés in the unit. However, several differential species of the submontane Cephalanthero-Fagetum were reported from the Muránska planina Mts (Brachythecium salebrosum, Dicranum scoparium, Isothecium myosuroides, Plagiochila porelloides, Plagiomnium rostratum and Sanionia uncinata; Kliment et al. 2010).
Effect of environmental conditions on understorey bryophyte richness, composition and trait pattern
Observed situation in calcareous beech forests of Central Slovakia where positive correlation between bryophyte species richness and cover of bare rocks was found supports the assumption that the availability of substrates promotes species richness (von Oheimb et al. 2007; Cole et al. 2008; Táborská et al. 2015; Kutnar et al. 2023). Similar positive relationship with dead wood amount reported by Dittrich et al. (2014) and Hofmeister et al. (2015) is apparent in the studied area, however, we did not estimate the cover of dead wood.
While differences in species composition of vascular plants are closely linked to the soil properties (Ellenberg et al. 1992), in bryophytes this relationship may not be so evident. This means that not all terrestrial bryophytes of studied calcareous beech forests have high EIV values for substrate reaction. Species indicating acidic situations (Calypogeia integristipula, Dicranella heteromalla, Polytrichum formosum) grow with basiphilous taxa in one community (Barbula unguiculata, Flexitrichum flexicaule, Homalothecium lutescens, Oxyrrynchium hians). In addition, many basiphilous (Ctenidium molluscum, Exsertotheca crispa, Fissidens dubius, Plagiochila porelloides) or rather generalistic species (Amblystegium serpens, Brachytheciastrum velutinum, Hypnum cupressiforme) also recorded frequently in the studied area have preferences for various other substrates besides soil (Frey et al. 2006; Hill et al. 2007). This is especially true for some acidophilous taxa (Dicranum scoparium, but also Cephalozia cuspidata, Lophocolea heterophylla, Plagiothecium curvifolium, P. laetum and Tetraphis pellucida, albeit they were recorded rarely) which are apparently indifferent to soil properties and they did not follow the composition of vascular plants in the understorey. The occurrence of acidophytes along with calciphilous epiliths was already reported by Kliment et al. (2010) from calcareous beech forests.
Our results show that heterogeneity of species traits is related to heterogeneity of substrates in the forest understorey. Besides terrestrial bryophytes, the complete set of species is supplemented greatly by those growing on rocks and wood. This is also evident from the ecological traits of all bryophyte species, where a significant percentage of them show a high preference for not only soil (including soil on rock, gravel or sand) but also different types of wood (living or decorticated) or rock (coarse and soft), either of basic or acidic reaction. A great variety of morphological traits and traits related to the life strategies of species implies the heterogeneity of available substrates. Appart from site properties, substrate heterogeneity is affected by forest management. Studies in beech-dominated forests of Central Europe (Ódor and Standovár 2001; Hofmeister et al. 2015; Kaufman et al. 2017; Kutnar et al. 2023) and Central Balkans (Sabovljević et al. 2010) have shown that bryophyte species richness in unmanaged primeval forests is higher than in managed stands. In our study, amount of dead wood varied according to management intensity. Nature reserves and unmanaged protected stands of steep slopes were frequent within the used dataset, however, managed productive stands were also involved. Absence of management supported higher amount of coarse woody debris in plots and enriched species composition by wood-inhabiting bryophytes. Beech logs can especially support species that form rough mats with lateral erect branches (Żarnowiec et al. 2021). Stefańska-Krzaczek et al. (2022) showed a positive correlation of old unmanaged forests with species occupying more than one substrate. They attributed this to the forest continuity and a higher availability of different substrates in such forests (especially decaying wood and trunks of old trees) which can thus promote both generalists and forest specialists preferring specific substrates. Unmanaged forests also promote the establishment of perennial and long-lived species typical for later successional stages and stable environments (according to During 1992). In the study by Stefańska-Krzaczek et al. (2022), perennial species were associated with coniferous forests which are assumed to have better light availability, less competition by vascular plants (Barbier et al. 2008) and no limitations by a high amount of deciduous litter (Startsev et al. 2008; Márialigeti et al. 2009; Jean 2017) and can thus support more species that colonize soil substrates (Caners et al. 2013). The occurrence of long-lived and perennial taxa in the studied beech forests of Central Slovakia was, most likely due to the presence of rocky outcrops (hence the correlation of species such as Ctenidium molluscum, Encalypta streptocarpa, Exsertotheca crispa and Fissidens dubius with the cover of bare rocks). It was shown by Virtanen and Oksanen (2007) that connected rock boulders have higher overall species richness of bryophytes than isolated ones but in their study the connectivity had stronger impact on richness of colonists than of perennial species. On the other hand, managed stands can harbour many species occupying disturbed and unstable habitats (according to During 1992) such as short-lived shuttle species which were found to prefer deciduous stands (Stefańska-Krzaczek et al. 2022).
In our study, most species in terms of their moisture indicator values are confined to moderately moist soils, regardless of their substrate preference, and therefore, species richness of bryophytes was negatively correlated with xericity. The highest values of xericity are in the south facing steep slopes with usually shallow soils and a relatively higher availability of light in the understorey. However, only Dicranum scoparium, Pseudoleskeella nervosa and partially Lophocolea heterophylla, Ptychostomum moravicum, Schistidium apocarpum or Tortella tortuosa (most of which are acrocarpous species) showed positive correlation with xericity, but these species are half-shade plants preferring mostly moderately moist soils and cooler climate. Lophocolea heterophylla is typical in spruce forests with cooler and moister microclimate compared to European beech stands (Nihlgård 1969). Similarly, Caners et al. (2013) found that coniferous forests support higher abundances of liverworts (several of which had smooth mat life form), acrocarpous mosses and species that have greater moisture requirements compared to mixed forests. Some liverworts (Cephalozia bicuspidata, Lophocolea heterophylla, Radula complanata) but also mosses (Plagiomnium cuspidatum, Plagiothecium spp.) forming smooth mats that according to Caners et al. (2013) should be related to higher moisture retention were recorded in our study (altough rarely) but they mostly show intermediate EIV values for moisture. Our findings are rather in accordance with Tinya et al. (2009) where the response of bryophytes to light, moisture and temperature did not fully agree with their EIV. Tinya et al. (2009) further showed in a deciduous-coniferous mixed woodland in Western Hungary that mainly terricolous, opportunistic and mineral soil-inhabiting species had significant positive correlations with light, whereas epiphytic and epixylic species did not. The later were much more influenced by the availability of the required substrate (bark of the adequate tree species or dead wood in the preferred decay stages). Even though bryophytes from tree trunks and root swellings were not collected in our study, many species growing frequently as epiphytes were captured on other substrates and they are important components of biodiversity in natural temperate forests.
Conclusion
Our results indicate that bryophyte species composition in studied beech forests is mostly influenced by moisture availability and the cover of bare rocks. Majority of bryophyte species of forest understorey recorded in our study grow on other substrates beside soil, especially on rocks, living and dead wood. Thus, the overall diversity of available substrates greatly contributes to the species richness of bryophytes and total phytodiversity of the forest community. Epiphytic, epixylic and epilithic bryophytes constitute a significant element of forest plant diversity, but are rarely studied by phytosociologists. Some species of bryophytes were proven as diagnostic species in syntaxonomical units of calcicolous beech forests. Therefore, for a complete picture of species composition and diversity of forest plant communities, and their proper differenciation, bryophyte sampling of all substrates and microhabitats involving soil, rock, dead wood and tree trunks is recommended.
Data Availability
Data used in the study are available upon request.
References
Alegro A, Šegota V, Rimac A, Papp B (2023) Diversity, ecology and phytogeography of bryophytes across temperate forest communities – insight from Mt. Papuk (Croatia, SE Europe). Plants 12:3346. https://doi.org/10.3390/plants12193346
Austin MP, Cunningham RB, Fleming PN (1984) New approaches to direct gradient analysis using environmental scalars and statistical curve-fitting procedures. Vegetatio 55:11–27. https://doi.org/10.1007/BF00039976
Barbier S, Gosselin F, Balandier P (2008) Influence of tree species on understory vegetation diversity and mechanisms involved – a critical review for temperate and boreal forests. For Ecol Manag 254:1–15. https://doi.org/10.1016/j.foreco.2007.09.038
Bernhardt-Römermann M, Poschlod P, Hentschel J (2018) BryForTrait – A life-history trait database of forest bryophytes. J Veg Sci 29(4):798–800. https://doi.org/10.1111/jvs.12646
Borhidi A (2003) Magyarország növénytársulásai. Akadémiai Kiadó, Budapest
Braun-Blanquet J (1964) Pflanzensoziologie – Grundzüge Der Vegetationskunde 3rd ed. Springer, Wien
Caners RT, Macdonald SE, Belland RJ (2013) Bryophyte assemblage structure after partial harvesting in boreal mixedwood forest depends on residual canopy abundance and composition. For Ecol Manag 289:489–500. https://doi.org/10.1016/j.foreco.2012.09.044
Chytrý M (2013) Vegetace České republiky 4. Academia, Praha
Chytrý M, Tichý L, Holt J, Botta-Dukát Z (2002) Determination of diagnostic species with statistical fidelity measures. J Veg Sci 13:79–90. https://doi.org/10.1111/j.1654-1103.2002.tb02025.x
Cole HA, Newmaster SG, Bell FW, Pitt D, Stinson A (2008) Influence of microhabitat on bryophyte diversity in Ontario mixedwood boreal forest. Can J Forest Res 38(7):1867–1876. https://doi.org/10.1139/X08-036
Dierßen K (2001) Distribution, ecological amplitude and Phytosociological characterization of European bryophytes J. Cramer Publishing Company, Stuttgart
Dittrich S, Jacob M, Bade C, Leuschner C, Hauck M (2014) The significance of deadwood for total bryophyte, lichen, and vascular plant diversity in an old-growth spruce forest. Plant Ecol 215(10):1123–1137. https://doi.org/10.1007/s11258-014-0371-6
During HJ (1992) Ecological classifications of bryophytes and lichens. In: Bates JW, Farmer AM (eds) Bryophytes and lichens in a changing environment. Clarendon Press, Oxford, pp 1–31
Ellenberg H, Weber HE, Düll R, Wirth V, Werner W, Paulißen D (1992) Zeigerwerte von Pflanzen in Mitteleuropa – Scripta Geobotanica. Verlag Erich Goltze KG, Göttingen
Fajmonová E (1971) Príspevok k fytocenológii vápencových bučín stredného Považia. Biológia 26(7):517–529
Fajmonová E (1972) Príspevok k fytocenológii vápencových bučín stredného Považia Carici albae(Abieti-) Fagetum Klika (1936) 1949. Biologia 27(1):31–42
Fajmonová E, Šimeková J (1973) Beitrag Zur Phytocenologischen Klassifikation Der Kalkstein-Buchenwälder in Der Westkarpaten. Acta Fac Rer Natur Univ Comen Bot 21:31–49
FAO (1988) Fao/Unesco Soil Map of the World. Revised legend with corrections and updates – World Soil Resources Report 60. FAO, Wageningen
Faško P, Šťastný P (2002) Priemerné ročné úhrny zrážok. In: MŽP, SAŽP (ed) Atlas krajiny SR, Ministerstvo životného prostredia SR, Slovenská agentúra životného prostredia SR, Bratislava, Banská Bystrica, p 99
Frey W, Frahm J-P, Fischer E, Lobin W (2006) The liverworts, mosses and ferns of Europe. Harley Books, Colchester
Friedel A, von Oheimb G, Dengler J, Härdtle W (2006) Species diversity and species composition of epiphytic bryophytes and lichens – a comparison of managed and unmanaged beech forests in NE Germany. Feddes Repert 117:172–185. https://doi.org/10.1002/fedr.200511084
Frisvoll AA, Prestø T (1997) Spruce forest bryophytes in central Norway and their relationship to environmental factors including modern forestry. Ecography 20:3–18
Fritz Ö, Heilmann-Clausen J (2010) Rot holes create key microhabitats for epiphytic lichens and bryophytes on beech (Fagus sylvatica). Biol Conserv 143:1008–1016. https://doi.org/10.1016/j.biocon.2010.01.016
Futák J (1943) Kremnické hory Mountains. Geo-botanical Studies. Matica Slovenská, Turčiansky Sv. Martin
Futák J (1984) Fytogeografické členenie Slovenska. In: Bertová L (ed) Flóra Slovenska IV/1. Veda, Bratislava, pp 418–419
Galvánek J (1999) The Banská Bystrica landscape – values, processes, changes and their effects on biodiversity. Acta Facultatis Rerum Naturalium Universitatis Matthiae Belii – Geographical studies 6:67–73
Grabherr G, Reiter K, Willner W (2003) Towards objectivity in vegetation classification: the example of the Austrian forests. Plant Ecol 169:21–34. https://doi.org/10.1023/A:1026280428467
Gustafsson L, Eriksson I (1995) Factors of importance for the epiphytic vegetation of aspen (Populus tremula) with special emphasis on bark chemistry and soil chemistry. J Appl Ecol 32:412–424. https://doi.org/10.2307/2405107
Hill MO, Preston CD, Bosanquet SD, Roy DB (2007) BRYOATT – attributes of British and Irish mosses, liverworts and Hornworts with Information on native status, size, life form, life history, geography and Habitat. CEH Publication, Huntingdon
Hodgetts NG, Söderström L, Blockeel TL et al (2020) An annotated checklist of bryophytes of Europe, Macaronesia and Cyprus. J Bryol 42(1):1–116. https://doi.org/10.1080/03736687.2019.1694329
Hofmeister J, Hošek J, Holá E, Novozámská E (2015) Decline in bryophyte diversity in predominant types of central European managed forests. Biodivers Conserv 24:1391–1402. https://doi.org/10.1007/s10531-015-0863-2
Hokkanen PJ (2004) Bryophyte communities in herb-rich forests in Koli, eastern Finland: comparison of forest classifications based on bryophytes and vascular plants. Ann Bot Fennici 41:331–365
Hrivnák R, Slezák M, Ujházy K, Máliš F, Blanár D, Ujházyová M, Kliment J (2019) Phytosociological approach to scree and ravine forest vegetation in Slovakia. Ann for Res 62(2):183–200. https://doi.org/10.15287/afr.2019.1355
Humphrey JW, Davey S, Peace AJ, Ferris R, Harding K (2002) Lichens and bryophyte communities of planted and semi-natural forests in Britain: the influence of site type, stand structure and deadwood. Biol Conserv 107:165–180. https://doi.org/10.1016/S0006-3207(02)00057-5
Jagodziński AM, Wierzcholska S, Dyderski MK, Horodecki P, Rusińska A, Gdula AK, Kasprowicz M (2018) Tree species effects on bryophyte guilds on a reclaimed post-mining site. Ecol Eng 110:117–127. https://doi.org/10.1016/j.ecoleng.2017.10.015
Janišová M (2001) Botanical research of the non-forest stands around the village of Riečka (Starohorské Vrchy mountain range). Bull Slov Bot Spol 23:121–129
Jasík M (1992) Mapping the orchid family (Orchidaceae) in Slovakia – the results from the environs of Banská Bystrica. Master thesis, Technical University in Zvolen
Jean M (2017) Effects of leaf litter and environment on bryophytes in boreal forests of Alaska. Dissertation, University of Saskatchewan
Kanka R, Turis P, Chilová V (2008) Phytosociological characteristic of the plant communities with the occurrence of endemic species Cyclamen fatrense. Hacquetia 7(1):21–31. https://doi.org/10.2478/v10028-008-0002-7
Kaufmann S, Hauck M, Leuschner C (2017) Comparing the plant diversity of paired beech primeval and production forests: management reduces cryptogam, but not vascular plant species richness. For Ecol Manag 400:58–67. https://doi.org/10.1016/j.foreco.2017.05.043
Király I, Nascimbene J, Tinya F, Ódor P (2013) Factors influencing epiphytic bryophyte and lichen species richness at different spatial scales in managed temperate forests. Biodivers Conserv 22:209–223. https://doi.org/10.1007/s10531-012-0415-y
Klika J (1927) Příspěvek Ke geobotanickému výzkumu Velké Fatry. Preslia 5:1–30
Klika J (1936) Das Klimax-Gebiet Der Buchenwälder in Der Westkarpathen [Beech climax area in Western Carpathinas]. Beih Bot Centralbl 55B:373–418
Kliment J, Ujházy K, Ujházyová M, Hrivnák R, Kochjarová J, Blanár D (2010) Syntaxonómia bukových a sutinových lesov južnej časti Muránskej planiny. Bull Slov Bot Spol 32(Supl. 2):161211
Kučera P (2010) Remarks to Abietion albae and its syntaxa. Acta Bot Univ Comen 45:3–12
Kutnar L, Kermavnar J, Sabovljević MS (2023) Bryophyte diversity, composition and functional traits in relation to bedrock and tree species composition in close-to-nature managed forests. Eur J for Res 142:1–18
Lee TD, La Roi GH (1979) Bryophyte and understory vascular plant beta diversity in relation to moisture and elevation gradients. Vegetatio 40:29–38. https://doi.org/10.1007/BF00052012
Lepš J, Šmilauer P (2003) Multivariate analysis of ecological data using CANOCO. Cambridge University Press, Cambridge
Marhold K, Hindák F (1998) Zoznam nižších a vyšších rastlín Slovenska: Checklist of non-vascular and vascular plants of Slovakia. VEDA, Bratislava
Márialigeti S, Németh B, Tinya F, Ódor P (2009) The effects of stand structure on ground-floor bryophyte assemblages in temperate mixed forests. Biodivers Conserv 18:2223–2241. https://doi.org/10.1007/s10531-009-9586-6
Martincová J, Ondrášek Ľ (2010) Grassland Monitoring of Meadows in the region around Banská Bystrica. Czech J Genet Plant Breed 46(Special Issue):S40–S44
Mikyška R (1939) Studie über die natürlichen Waldbestände Im Slowakischen Mittelgebirge (Slovenské Stredohorie). Ein Beitrag Zur Soziologie Der Karpatenwälder. Beih Bot Cbl 59:169–244
Mills SE, MacDonald SE (2004) Predictors of moss and liverwort species diversity of microsites in conifer dominated boreal forest. J Veg Sci 15:189–198. https://doi.org/10.1111/j.1654-1103.2004.tb02254.x
Mills SE, MacDonald SE (2005) Factors influencing bryophyte assemblage at different scales in the Western Canadian boreal forest. Bryologist 108:86–100. https://doi.org/10.1639/0007-2745(2005)108[86:fibaad]2.0.co;2
Moora M, Daniell T, Kalle H, Liira J, Pussa K, Roosaluste E, Opik M, Wheatley R, Zobel M (2007) Spatial pattern and species richness of boreonemoral forest understorey and its determinants – a comparison of differently managed forests. For Ecol Manag 250:64–70. https://doi.org/10.1016/j.foreco.2007.03.010
Mucina L, Bültmann H, Dierßen K et al (2016) Vegetation of Europe: hierarchical floristic classification system of vascular plant, bryophyte, lichen, and algal communities. Appl Veg Sci 19:3–264. https://doi.org/10.1111/avsc.12257
Nihlgård B (1969) The microclimate in a beech and a spruce forest – a comparative study from Kongalund, Scania, Sweden. Bot Notiser 122:333–352. https://doi.org/10.2307/3543854
Ódor P, Standovár T (2001) Richness of bryophyte vegetation in near-natural and managed beech stands: the effects of management-induced differences in dead wood. Ecol Bull 49:219–229. https://doi.org/10.2307/20113278
Puglisi M, Kürschner H, Privitera M (2013) Syntaxonomy, life forms and life strategies of the bryophyte vegetation of the Carnic Alps (NE Italy). Nova Hedwigia 96(3–4):325–349. https://doi.org/10.1127/0029-5035/2013/0081
Puglisi M, Kürschner H, Privitera M (2016) The mountain and high-mountain bryophyte vegetation of the Pollino National Park (Southern Italy): syntaxonomy and ecology. Nova Hedwigia 103(3–4):385–413. https://doi.org/10.1127/nova_hedwigia/2016/0362
Rambo TR, Muir PS (1998) Bryophyte species associations with coarse woody debris and stand ages in Oregon. Bryologist 101:366–376. https://doi.org/10.2307/3244175
Rydin H (2009) Population and community ecology of bryophytes. In: Goffinet B, Shaw AJ (eds) Bryophyte Biology, 2nd edn. Cambridge University Press, Cambridge
Sabovljević M, Vujičić M, Sabovljević A (2010) Diversity of saproxylic bryophytes in old-growth and managed beech forests in the central Balkans. Plant Biosyst 144(1):234–240. https://doi.org/10.1080/11263500903561015
Schumacher A (2000) Die Ökologie der Moose in mitteleuropäischen Buchenwäldern unter dem Einfluß der Forstwirtschaft. Diss Bot 331:1–176
Simmel J, Ahrens M, Poschlod P (2021) Ellenberg N values of bryophytes in Central Europe. J Veg Sci 32:e12957. https://doi.org/10.1111/jvs.12957
Slezák M, Hrivnák R, Ujházy K, Ujházyová M, Máliš F, Petrášová A (2016) Syntaxonomy and ecology of acidophilous beech forest vegetation in Slovakia. Phytocoenologia 46(1):69–88
Smith AJE (ed) (1982) Bryophyte Ecology. Springer, Dordrecht
Startsev N, Lieffers VJ, Landhausser SM (2008) Effects of leaf litter on the growth of boreal feather mosses: implication for forest floor development. J Veg Sci 19:253–260. https://doi.org/10.3170/2008-8-18367
Šťastný P, Nieplová E, Melo M (2002) Priemerná ročná teplota vzduchu. M 1: 2 000 000, p. 98. In: MŽ, SAŽP (ed) Atlas krajiny Slovenskej republiky. Ministerstvo životného prostredia SR, Slovenská agentúra životného prostredia SR, Bratislava, Banská Bystrica, pp 99–344
StatSoft (2013) STATISTICA 12. http://www.statsoft.cz/. Accessed 23 August 2023
Stefańska-Krzaczek E, Swacha G, Żarnowiec J, Raduła MW, Kącki Z, Staniaszek-Kik M (2022) Central European forest floor bryophytes: richness, species composition, coexistence and diagnostic significance across environmental gradients of forest habitats. Ecol Ind 139:108954. https://doi.org/10.1016/j.ecolind.2022.108954
Świerkosz K, Reczyńska K, Pech P, Kuras I, Hédl R (2018) Syntaxonomy and ecology of beech forest vegetation in southwestern Poland. Phytocoenologia 48(3):297–320. https://doi.org/10.1127/phyto/2018/0248
Táborská M, Přívětivý T, Vrška T, Ódor T (2015) Bryophytes associated with two tree species and different stages of decay in a natural fir-beech mixed forest in the Czech Republic. Preslia 87:387–401
Thomsen RP, Svenning JC, Balslev H (2005) Overstorey control of understorey species composition in a nearnatural temperate broadleaved forest in Denmark. Plant Ecol 181:113–126. https://doi.org/10.1007/s11258-005-3996-7
Tichý L (2002) JUICE, software for vegetation classification. J Veg Sci 13:451–453. https://doi.org/10.1111/j.1654-1103.2002.tb02069.x
Tichý L, Chytrý M (2006) Statistical determination of diagnostic species for site groups of unequal size. J Veg Sci 17:809–818. https://doi.org/10.1111/j.1654-1103.2006.tb02504.x
Tinya F, Márialigeti S, Király I, Németh B, Ódor P (2009) The effect of light conditions on herbs, bryophytes and seedlings of temperate mixed forests in Őrség, Western Hungary. Plant Ecol 204:69–81. https://doi.org/10.1007/s11258-008-9566-z
Turis P (2001) The flax species (Linum) in the northern part of Zvolenská kotlina basin. In: Turisová I (ed) Proc Conf Ecological Diversity of the Model site of Banská Bystrica region. ŠOP SR, UMB, Stredoslovenské múzeum, Banská Bystrica, pp 171–180
Turisová I, Martincová E (2001) Flora in the area of Banská Bystrica. In: Turisová I (ed) Proc. Conf. Ecological Diversity of the Model site of Banská Bystrica region, ŠOP SR, UMB, Stredoslovenské múzeum, Banská Bystrica, pp 107–123
Tzonev R, Dimitrov M, Chytrý M, Roussakova V, Dimova D, Gussev C, Pavlov D, Vulchev V, Vitkova A, Gogoushev G, Nikolov I, Borisova D, Ganeva A (2006) Beech forest communities in Bulgaria. Phytocoenologia 36(2):247–279. https://doi.org/10.1127/0340-269X/2006/0036-0247
Uhliarová E, Martincová E (2004) Flora of the non-forest areas near the village of Badín. In: Turisová I, Prokešová R (ed) Proc. Conf. Ecological Diversity of Zvolenská kotlina Basin. ŠOP SR, UMB, Stredoslovenské múzeum, Banská Bystrica, pp 113–119
Ujházyová M, Ujházy K, Máliš F (2013) Bukové lesy juhozápadnej časti Veľkej Fatry. Bull Slov Bot Spol 35(2):161–198
Ujházyová M, Ujházy K, Máliš F, Slezák M, Hrivnák R (2021) Syntaxonomical revision of the order Fagetalia Sylvaticae Pawlowski ex Pawlowski et al. 1928 in Slovakia. Biologia 76:1929–1968. https://doi.org/10.2478/s11756-020-00661-1
Valachovič M, Hegedušová Vantárová K, Kanka R, Kliment J, Kollár J, Máliš F, Piscová V, Senko D, Slezák M, Ujházy K, Ujházyová M, Žarnovičan H (2014) Lesné spoločenstvá Pohoria Vihorlat (Východné Slovensko). Phytopedon (Bratislava) –. J Soil Sci 13(1):13–41
Virtanen R, Oksanen J (2007) The effects of habitat connectivity on cryptogam richness in boulder metacommunity. Biol Conserv 135:415–422. https://doi.org/10.1016/j.biocon.2006.10.013
Vitt DH, Li Y, Belland R (1995) Patterns of bryophyte diversity in peatlands of continental western Canada. Bryologist 98:218–227. https://doi.org/10.2307/3243306
von Oheimb G, Friedel A, Bertsch A, Härdtle W (2007) The effects of windthrow on plant species richness in a central European beech forest. Plant Ecol 191:47–65. https://doi.org/10.1007/s11258-006-9213-5
Westhoff V, van der Maarel E (1978) The Braun-Blanquet approach. In: Whittaker RH (ed) Classification of plant communities, 2nd edn. Junk, The Hague, pp 287–399
Willner W, Grabherr G (eds) (2007) Die Wälder und Gebüsche Österreichs: Ein Bestimmungswerk Mit Tabellen. Spektrum Akademischer Verlag, Heidelberg
Willner W, Jiménez-Alfaro B, Agrillo E, Biurrun I et al (2017) Classification of European beech forests: a Gordian knot? App Veg Sci 20:494–512. https://doi.org/10.1111/avsc.12299
Żarnowiec J, Staniaszek-Kik M, Chmura D (2021) Trait-based responses of bryophytes to the decaying logs in central European mountain forests. Ecol Indic 126:107671. https://doi.org/10.1016/j.ecolind.2021.107671
Acknowledgements
The authors would like to thank Jan Kučera and Svatava Kubešová for revision of some bryophyte specimens and Marek Kotrík for the map design. The paper was supported by the Scientific Grant Agency of the Slovak Republic under Grant VEGA 1/0624/21.
Funding
Open access funding provided by The Ministry of Education, Science, Research and Sport of the Slovak Republic in cooperation with Centre for Scientific and Technical Information of the Slovak Republic
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Širka, P., Ujházyová, M. & Ujházy, K. Bryophytes in classification and ecology of calcareous beech forests in Central Slovakia. Biologia 79, 1209–1223 (2024). https://doi.org/10.1007/s11756-023-01570-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11756-023-01570-9