Abstract
Hares (genus Lepus) are widely distributed in Europe, and they are adapted to various types of habitats. Many species are known to hybridise, and previous molecular genetic studies have revealed recurrent introgressions between species at all stages of the genus’s radiation. The Don hare (Lepus tanaiticus) was described from the Late Pleistocene of the Southern Urals and subsequently reported from coeval deposits of various regions of northern Eurasia. It is morphologically close to the mountain hare (Lepus timidus) and recent studies of mitochondrial DNA questioned its status as an independent species. Here we compare cytochrome b and control-region sequences of mtDNA of arctic Lepus, including, for the first time, eight specimens from Late Pleistocene localities of Ukraine, in order to analyse the phylogenetic relationships between representatives of different taxa. The phylogenetic tree and haplotype network analyses do not support the taxonomic distinctness of the Don hare, and only specimens of Lepus arcticus and Lepus othus form monophyletic groups based on the control-region sequences. Instead, L. tanaiticus are scattered among specimens of L. timidus. The obtained results support the hypothesis that the Don hare is an ancient morphotype of L. timidus, and its distinctive morphological traits are the result of increased geographical variation of the latter due to range expansion and adaptation to the specific conditions of the periglacial biome, similarly to other Late Pleistocene small-mammal species.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hares (genus Lepus Linnaeus, 1758) are represented in the modern fauna by 32 species, five of which are distributed in Europe (Smith et al. 2018). Molecular genetic studies suggest that the genus Lepus most probably originated in North America ca. 12 Myr ago and later dispersed across other continents via an ancestral lineage that crossed the Bering Strait to Asia about 7–5 Myr ago (Matthee et al. 2004). The geographic ranges of modern European species of hares partly overlap, and hybridisation is quite common in this group (Alves et al. 2003, 2008; Melo-Ferreira et al. 2005), possibly having an adaptive effect (Thulin et al. 1997, 2006; Ferreira et al. 2021; Pohjoismaki et al. 2021). Systematic treatments of lagomorphs in general are often controversial because of not only hybridisation and introgression, but also rapid radiation and local adaptations to a variety of habitats (Melo-Ferreira et al. 2012), which resulted in high intraspecific variability of widespread species. Molecular phylogenetic research on leporids has also provided conflicting results in relation to data inferred from morphology-based analyses (Robinson and Matthee 2005). Previous studies have revealed recurrent introgressions at all stages of the Lepus radiation, which resulted in genetic connections among all major clades of Eurasian hares (e.g., Thulin et al. 1997, 2006; Alves et al. 2003, 2006; Melo-Ferreira et al. 2005, 2007, 2012; Fredsted et al. 2006; Ahlgren et al. 2016; Ferreira et al. 2021).
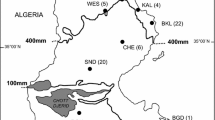
Remains of hares are known from a number of Pleistocene and Early Holocene localities in the territory of Ukraine (Fig. 1). They were usually identified as any of three species — the brown hare Lepus europaeus Pallas, 1778, the mountain hare Lepus timidus Linnaeus, 1758, or the extinct Don hare Lepus tanaiticus Gureev, 1964 (Gromov 1961; Rekovets 1985, 1994, 1995; Rekovets and Topachevsky 1988; Averianov 1994, 1998, 2001; Krokhmal’ and Rekovets 2010). The latter was erected based on morphological characters of a right mandible from the Late Pleistocene of the Southern Urals. According to the original description, L. tanaiticus is also present in the fossil record of the Novgorod-Siverskyi locality in Ukraine and other Late Pleistocene and Early Holocene faunal assemblages of Eastern Europe and Northern Asia (Rekovets 1985, 1995; Averianov 1995, 1998, 2001; Kosintsev 2007; Prost et al. 2010; Sharko et al. 2023).
Pleistocene and Holocene record localities of hares in the territory of Ukraine (after Krokhmal’ and Rekovets 2010, modified after Krokhmal’ et al. 2021, 2022; Gorobets et al. 2023): 1 — Melekino; 2 — Kairy; 3 — Lymany; 4 — Ushkalka; 5 — Cherevychne; 6 — Luzanivka; 7 — Karai-Dubina; 8 — Bilshovyk; 9 — Protopopovka; 10 — Syniakovo; 11 — Ozerne; 12 — Morozivka; 13 — Krasnosilka; 14 — Gunky; 15 — Demydivka; 16 — Stari Kodaky; 17 — Kiik-Koba; 18 — Chokurcha; 19 — Kabazi; 20 — Gintsi (Gontsy); 21 — Molodova; 22 — Adzhi-Koba; 23 — Syuren 1; 24 — Buran-Kaya; 25 — Nyzhnie Kryvche; 26 — Novgorod-Siverskyi; 27 — Anetivka; 28 — Mezhyrich; 29 — Pushkari; 30 — Amvrosiivka; 31 — Dobranichivka; 32 — Chulativ; 33 — Mizyn (Mezin); 34 — Divochi Skeli; 35 — Osokorivka; 36 — Proshchalnaya; 37 — Alymivskyi Navis; 38 — Murzak-Koba; 39 — Syuren 2; 40 — Shan-Koba; 41 — Mala Ugolka; 42 — Raspopyntsi. Location numbers of the samples analysed in the study are indicated in bold.
The morphological traits that distinguish L. tanaiticus as an independent species include its large body size (10% exceeding that of L. timidus) and features of dental morphology, particularly the high dental portion of the mandible, especially on p3). It has, however, been argued that body size and dental morphology are highly adaptive traits and thus poor markers for species assignment (see Prost et al. 2010), and the species status of L. tanaiticus has repeatedly been questioned. A comparative phylogenetic analysis of L. tanaiticus and extant species of the arctic hare group (Lepus arcticus Ross, 1819, Lepus othus Merriam, 1900, and L. timidus) based on a mitochondrial D-Loop fragment provided no reliable support for the species status of the Don hare, and it was suggested to be a distinct morphotype of L. timidus (Prost et al. 2010). Sharko et al. (2023) analysed the mitochondrial genome of Pleistocene Don hares from north-eastern Eurasia and revealed that specimens of L. tanaiticus younger than 39 ka BP are phylogenetically close to L. timidus, whereas older specimens form a distinct mitochondrial clade. The authors speculated that the genetic proximity between ‘young’ Don hares and the mountain hare is a result of hybridisation that occurred at the end of the Late Pleistocene.
Phylogenetic studies of L. tanaiticus have usually included specimens from localities in the territory of Russia, but specimens assigned to this species and recovered from other parts of Europe have never been analysed before using molecular genetic approaches. The aim of our study was to carry out a comparative analysis of mitochondrial DNA sequences of representatives of the arctic hare group, including, for the first time, specimens of L. tanaiticus recovered from Late Pleistocene localities of Ukraine, and contribute to the understanding of its taxonomic status and relationship to other species of arctic hares.
Materials and methods
The specimens considered (mandible fragments and parts of the postcranial skeleton) represent the remains of eight individuals of the genus Lepus recovered from four localities in the territory of Ukraine dated to 16,000–20,000 years BP (Table 1). They are housed in the Department of Palaeontology at the National Museum of Natural History, National Academy of Sciences of Ukraine (Kyiv). These specimens were assigned to Lepus tanaiticus (n = 5) and Lepus sp. (n = 3) based on morphological characters (Gureev 1964; Rekovets 1985; Rekovets and Topachevsky 1988).
The molecular analysis was carried out in the Department of Genetics at Wrocław University of Life Sciences and in the Institute of Genetics and Biotechnology of the University of Warsaw (Poland). The phenol-chloroform method (Bilton and Jaarola 1996; Capelli and Tschentscher 2005) accompanied with three additional methods — with Dextran Blue (Kalmar et al. 2000), silica (Höss and Pääbo 1993; Rohland and Hofreiter 2007) and Chelex (Walsh et al. 1991) — was applied to extract the mtDNA from the specimens considered.
Ancient DNA is usually highly fragmented thus it is recommended to use primer pairs for a few relatively short fragments (Poinar 2003), product length ca. 80–200 bp (Table 2). In order to verify the results, at least three replications of the PCR and sequencing reactions were performed for each reaction. Five short overlapping fragments with a total length of about 300 bp were used for the amplification of the control-region sequence (Prost et al. 2010).
For the amplification of the cytochrome b sequence, five overlapping fragments with a total length of about 300 bp were used (Smith et al. 2017). In each case, the PCR was prepared in a volume of 25 µl. It was performed with 2.5 µl of PCR buffer, 0.5 µl of each primer (10 µM), 0.5 µl of dNTP (10 µM), 1 µl of MgCl (2.5 mM), 2.5 µl of BSA (1 mg/ml), 0.4 µl of Hot Start polymerase (Qiagen, Germany), distilled water, and the appropriate volume of DNA depending on the quality and concentration of the template. The mixtures were amplified in the Eppendorf Mastercycler EP thermocycler using the following program: 95 °C — 10 min, then 45 cycles (for CR sequences) and 55 cycles (for CytB) [94 °C — 30 s., 48–50 °C (in the case of CR) or 50–51 °C (in the case of CytB sequences) (depending on the primer) — 30 s., 72 °C — 30 s.], 72 °C — 7 min, 12 °C ‘for ever’. PCR products were purified with Exo/SAP (USB Corp.) and sequenced using ABI BigDye v3.1 terminators (Applied Biosystems) on an ABI 3730XL sequencer at the sequencing facility of the Institute of Biochemistry and Biophysics, Polish Academy of Sciences in Warsaw (Poland).
Three replicates of the PCR for each specimen and each primer were aligned and a consensus sequence was constructed. Then, the sequences from both primers for the same DNA fragment were aligned and a consensus sequence was created for a given primer pair in BioEdit software (Hall 1999). The sequences of all fragments were aligned into the final consensus sequence. The sequences obtained in this way for all individuals, combined with those obtained from GenBank database, were aligned using the Muscle algorithm (Edgar 2004) implemented in Seaview (Gouy et al. 2010). The obtained sequences were compared with the available control-region sequences of L. tanaiticus (Prost et al. 2010) and control-region and cytochrome b sequences of selected representatives of the genus Lepus from GenBank database (Halanych et al. 1999; Pierpaoli et al. 1999; Waltari et al. 2004; Kasapidis et al. 2005; Waltari and Cook 2005; Wu et al. 2005; Melo-Ferreira et al. 2007, 2012; Prost et al. 2010; Ramirez-Silva et al. 2010) (Tables 3 and 4).
After alignment, the analysed sequences were truncated in order to obtain a compact block of DNA. The sets of sequences prepared in this way were used to create phylogenetic trees using Maximum Likelihood (ML) and Bayesian Analysis (BA) methods. For analyses with the ML method, the PHYML program was used with the implemented smart model selection option for automatic selection of the substitution model (Guindon et al. 2010). The tree topology was verified with 1000 bootstrap replicates. The MrBayes 3.2 software was used to create the trees using the Bayesian method (Ronquist et al. 2012). The jModelTest 2.1.10 application was used to estimate the best-fit nucleotide substitution models used in the MrBayes program (Darriba et al. 2012). For protein coding sequences (CytB), separate models were selected for each codon position — JC, HKY, and K2P (K80) for I, II, and III position of the codon, while a single general model (HKY + I + G) was selected for the control-region sequence. During the analysis in the MrBayes programme, we used the option of two independent runs, each consisting of four Markov chains. The trees were sampled every hundredth generation for 20 000 000 generations (using 25% burn-in values). The analysis was completed when the average standard deviation of split frequencies for both runs stabilised at a level below 0.01. In many cases, standard tree-based phylogenetic analysis is unable to fully resolve close phylogenetic relationships; therefore, a haplotype network was created for individual sequences. The Median Joining method (Bandelt et al. 1999) implemented in the PopART 1.7 programme (Leigh and Bryant 2015) was used to create the network. For their implementation, the same data sets were used as in the case of particular phylogenetic trees.
Results
The obtained sequences combined with data from previous molecular analyses allowed the construction of phylogenetic trees and haplotype networks to study the genetic relationships among the hares considered. We suggest that the position of Lepus individuals from the Late Pleistocene of Ukraine in the phylogenetic tree based on the cytochrome b and control-region sequence analyses is reliable, considering the high posterior probability level and support of all nodes (Figs. 2 and 3).
Phylogenetic tree of the genus Lepus based on the analysis of cytochrome b mtDNA sequences. Bayesian posterior probabilities (BPP) and NJ bootstrap values are shown at each branching point (BBP ⁄ NJ bootstraps). Branches indicated with an asterisk are not supported under the 70% majority consensus in the NJ tree. Sequences highlighted in bold belong to the specimens from Ukraine (L. tanaiticus, Lepus sp.). Sequences of L. europaeus were used as outgroup
Cytochrome b sequence analysis
The cytochrome b mtDNA sequences were obtained from four individuals of the genus Lepus whose remains were recovered from Mizyn and Gintsi. They were compared with the respective sequences of extant members of the arctic hare group (L. timidus, L. arcticus, and L. othus) now distributed in Northern Eurasia, Central Europe, and North America. Two distinct clades were obtained with high support of nodes; a polytomic clade A including the majority of analysed specimens and clade B represented by a single L. timidus from western Russia (Fig. 2). Within clade A, three sub-groups can also be noted comprising specimens of L. timidus from the Alps and eastern Russia together with those from Mizyn and Gintsi (A1), specimens from Norway (A2) and the Urals (A3). Otherwise, however, representatives of L. timidus are scattered in the tree. The individuals of L. arcticus and L. othus do not form clades separate from L. timidus. Two individuals from Mizyn and Gintsi form their own branch with a high Bayesian support (0.88). The sequences of two other individuals from Gintsi are located separately, in a different part of the tree (Fig. 2).
The cytochrome b sequences ranging in length from 290 to 364 bp and those from GenBank were used to build the haplotype network of the analysed Lepus species (Fig. 4). As in the case of the phylogenetic tree, there are two groups recognisable in the network. Group 1 consists of representatives of the brown hare, whereas all other individuals considered (L. arcticus, L. othus, and L. timidus from GenBank as well as L. tanaiticus from Mizyn and Lepus sp. from Gintsi) are placed in the second group with a star-like topology. Group 2 consists of a single haplotype located in the centre, which was the most common among all the analysed individuals, and six other haplotypes (Fig. 4).
Haplotype network of species of the genus Lepus based on the analysis of cytochrome b mtDNA sequences. The size of the nodes is proportional to their haplotype frequency. Missing intermediates are indicated by black dot. The lengths of the branches are proportional to the number of substitutions separating the haplotypes
Control-region sequence analysis
The control-region mtDNA sequences of hares ranging in length from 115 to 332 bp were analysed separately. They were obtained from seven individuals of the genus Lepus from Mezhyrich, Mizyn, Novgorod-Siverskyi, and Gintsi. The respective sequences of Lepus arcticus, L. europaeus, L. othus, and L. timidus from GenBank were used for comparison. In addition, five sequences indicated as L. t. tanaiticus by Prost et al. (2010) were also considered.
Both Bayesian and NJ tree reconstructions resulted in a consistent tree topology with ten (A–J) recognisable clades (Fig. 3). The clades A to F have a moderate Bayesian support. A single specimen of L. timidus from eastern Russia was the earliest to emerge on the tree, forming its own clade J, followed by other specimens of L. timidus and L. t. tanaiticus from various geographical localities, scattered from clade B to clade I. The clade B also includes specimens of L. othus, which is now restricted to westernmost Alaska, forming a separate sub-group (B1), while the rest of the specimens in the clade come from Russia, Norway, and Eastern Europe (B2–B3). In contrast to the tree based on CytB sequences, the North American L. arcticus forms a well-separated clade (A). The next three clades (C, D, and E) are formed by individuals of the mountain hare from Eastern Europe and Russia (Fig. 3). The specimen of L. tanaiticus from Mizyn is placed within the clade D, while another one from Novgorod-Siverskyi forms its own clade F. Such a distribution suggests some distinctness between their sequences. Clade G includes four specimens of Pleistocene hares from all of the studied Ukrainian localities. One individual from Gintsi (identified as Lepus sp.) together with L. t. tanaiticus are nested in the clade H. The clade I includes the sequences of two individuals of L. timidus from Sweden (Fig. 3).
The haplotype network of the control-region sequences (Fig. 5) resembles the distribution of specimens in the phylogenetic tree. The haplotypes of L. othus form a separate cluster (Group 1) showing greater differences in sequences compared to Group 2, which consists of sequences of L. timidus and two those of L. t. tanaiticus sensu Prost et al. (2010). Group 2 is connected with a dispersed series of haplotypes of L. timidus, L. tanaiticus from Mezhyrich, Mizyn, and Novgorod-Siverskyi, Lepus sp. from Gintsi, and three haplotypes of L. t. tanaiticus (Fig. 5). A separate group of haplotypes is formed by the sequences of L. arcticus (Group 3), although two specimens of this species fell outside this group. It is characterised by a high number of substitutions compared to other species.
Discussion
Molecular genetic techniques, especially the analysis of ancient DNA extracted from collection specimens, have revolutionised taxonomic studies and allow clarifying the relationships between extinct and extant species. Fossil taxa have traditionally been erected based on detailed morphological descriptions, although the range of variation of those diagnostic traits often remains poorly understood, especially when the number of available specimens is limited. Palaeogenetic studies allow a deeper and more accurate analysis of phylogenetic relationships and thus have the potential to resolve contradicting morphology-based conclusions. It is especially relevant when estimating past taxonomic diversity of various geographical regions and epochs in the context of major extinction events. The Late Pleistocene glaciation had a major impact on the evolution of numerous mammalian groups, including the lagomorphs that had played an important ecological role in periglacial ecosystems, although genetic and morphological data often contradict when analysing the phylogenetic relationships among various members of this group (e.g., Prost et al. 2010; Rabiniak et al. 2023).
The Don hare (Lepus tanaiticus) — a Late Pleistocene hare of the Palaearctic — is one of those dubious taxa, the systematic position of which has been controversial and remains unresolved (Prost et al. 2010; Sharko et al. 2023). The Don hare went extinct in the Holocene and most of its remains have been reported from the territory of present-day Russia. A large sample of hares from the Late Pleistocene of Ukraine, however, was also identified by Rekovets (1985) as L. tanaiticus based on characters described by Gureev (1964). At the same time, Rekovets (1985) amended the diagnosis of L. tanaiticus by adding a few additional characters, in particular a shortened diastema of the lower jaw, a more elongated palate, and more strongly developed zygomatic processes of the upper jaw. Although Rekovets (1985) treated the Don hare as an independent species, he also highlighted the substantial morphological similarity between L. tanaiticus and L. timidus and hypothesised that they could be phylogenetically close or even conspecific.
Previous studies of mtDNA sequences of arctic hares have revealed that L. tanaiticus falls within the range of variation of the extant L. timidus and phylogenetic tree analyses do not support the monophyly of the Don hare (Prost et al. 2010; Sharko et al. 2023). In this study, the analysis of cytochrome b and control-region sequences of extant arctic hares and specimens assigned to L. tanaiticus, including those from Late Pleistocene localities of Ukraine and first analysed genetically, has provided similar results: the Don hare is scattered in the obtained phylogenetic trees and haplotype networks among specimens of other species. Based on the control-region sequences, only specimens of L. arcticus (distributed in northern Canada and Greenland) and L. othus (distributed in western Alaska) form monophyletic groups (see Fig. 3). At the same time, L. othus is closer to L. timidus, which can be a sign of more recent introgression between these two species in periods when the Bering land bridge opened.
Genes of L. timidus are present in European hare species because of repeated introgressions in the past (Alves et al. 2003; Melo-Ferreira et al. 2005, 2007, 2012; Fredsted et al. 2006; Thulin et al. 1997, 2006; Ferreira et al. 2021), but molecular genetic data do not support the idea of phylogenetic distinctness of L. tanaiticus. Instead, it seems more likely that the Don hare was a distinct morphotype of L. timidus, as Prost et al. (2010) suggested earlier.
It is now commonly accepted that one should be careful when inferring the evolutionary history of species solely based on a limited number of genetic markers. Complete mitochondrial and nuclear genome analyses are therefore considered to provide more reliable implications for mammalian population genetics and phylogeny (e.g., Moska et al. 2016; Westerman et al. 2016; Urantowka et al. 2017). Complete mtDNA sequences were obtained for a number of Palaearctic Lepus species (e.g., see Ding et al. 2016; Giannoulis et al. 2018; Sharko et al. 2023), whereas the number of studies of whole-genome sequences of hares, particularly of L. timidus, is limited (e.g., Marques et al. 2020; Michell et al. 2022). Our study provides new valuable mtDNA data that both support previous findings of genetic and taxonomic research and supply new, important genetic information for further phylogenetic studies of Lepus species.
Nonetheless, the phylogenetic position of L. tanaiticus as a separate morphotype rather than an independent species inferred from the analysis of mtDNA fragments is remarkably similar to that of some other Late Pleistocene morphology-based small-mammal taxa. Rekovets (1985) considered the Don hare a common representative of the Late Pleistocene periglacial fauna of Eastern Europe, together with Citellus severskensis Gromov, 1958, Citellus superciliosus (Cuvier, 1825), Dicrostonyx gulielmi (Sanford, 1870), and Ochotona spelaea (Owen, 1846). Molecular genetic studies, however, questioned the species status of several Late Pleistocene mammalian taxa that were described based on morphological traits. The most recent examples are the pika species O. spelaea (Rabiniak et al. 2023) and the vole species Microtus bifrons Jeannet and Fontana, 2015 (Nadachowski et al. 2023), which turned out to be ancient morphotypes of the extant species Ochotona pusilla (Pallas, 1769) and Microtus arvalis (Pallas, 1778), respectively.
The main feature of the Late Pleistocene was glaciation, which also affected biome shifts and substantial range dynamics. The periglacial tundra expanded to the south along with the geographical range of species associated with this biome, whereas the range of temperate species also shifted, and they survived the periods of glaciation in southern refugia (e.g., see Jones et al. 2020; Sommer 2020). Consequently, range expansion could have increased the geographical variation of northern species and the appearance of well-distinguished morphotypes with adaptations to the specific conditions of the vast tundra steppe (Lister et al. 1987; Stewart 1999; Meiri et al. 2013; Lagerholm et al. 2017; Rabiniak et al. 2023). As a result, ecomorphological traits have been given taxonomic significance by researchers and distinct morphotypes were recognised as independent taxa or separate subspecies. Although, Rabiniak et al. (2023) argue that defining separate subspecies based on morphological data cannot be sustained by phylogenetic analysis as they are too closely related to extant representatives. Respectively, we can expect that morphological characters that were used to describe the Don hare as a separate species are also overestimated and are within the range of geographical variation that characterised L. timidus during the Late Pleistocene.
In the light of recent findings, the taxonomic status of various representatives of the Late Pleistocene vertebrate fauna will likely be subject to revision, which, in turn, will have a crucial role in the estimates of the impact of the Quaternary extinction event on local and global biodiversity (Barnosky et al. 2004). For instance, it was already suggested that the Pleistocene/Holocene transition in the Crimea, Ukraine, was not as drastic in terms of faunal turnover as in other parts of Eastern Europe (Benecke 1999; Kovalchuk et al. 2021). It is therefore possible that the Quaternary extinction (at least of small mammals) occurred at a much smaller scale and mainly involved ancient morphotypes of extant species.
References
Ahlgren H, Norén K, Angerbjörn A, Lidén K (2016) Multiple prehistoric introductions of the mountain hare (Lepus timidus) on a remote island, as revealed by ancient DNA. J Biogeogr 43(9):1786–1796. https://doi.org/10.1111/jbi.12759
Alves PC, Ferrand N, Suchentrunk F, Harris DJ (2003) Ancient introgression of Lepus timidus mtDNA into L. granatensis and L. europaeus in the Iberian Peninsula. Mol Phylogenet Evol 27(1):70–80. https://doi.org/10.1016/S1055-7903(02)00417-7
Alves PC, Harris D, Melo-Ferreira J, Branco M, Suchentrunk F, Boursot P, Ferrand N (2006) Hares on thin ice: introgression of mitochondrial DNA in hares and its implications for recent phylogenetic analyses. Mol Phylogenet Evol 40(2):640–641. https://doi.org/10.1016/j.ympev.2006.02.016
Alves PC, Melo-Ferreira J, Freitas H, Boursot P (2008) The ubiquitous mountain hare mitochondria: multiple introgressive hybridization in hares, genus Lepus. Philos Trans R Soc Lond B 363:2831–2839. https://doi.org/10.1098/rstb.2008.0053
Averianov AO (1994) Pleistocene hares (Lepus, Lagomorpha) from the Crimea. Tr Zool Inst Ross Akad Nauk (St Petersburg) 256:69–91 [In Russian]
Averianov AO (1995) Late Pleistocene hare, Lepus tanaiticus (Lagomorpha, Leporidae) of Siberia. In: Baryshnikov GF (ed) Studies of Pleistocene and Recent Mammals. Proc Zool Inst 263, pp 121–162. [In Russian]
Averianov AO (1998) Late pleistocene hares (Lepus) of the russian plain. Ill State Mus Sci Papers 27:41–68
Averianov AO (2001) Lagomorphs (Mammalia) from the Pleistocene of Eurasia. Paleontol J 35(2):191–199
Bandelt HJ, Forster P, Röhl A (1999) Median-joining networks for inferring intraspecific phylogenies. Mol Biol Evol 16(1):37–48. https://doi.org/10.1093/oxfordjournals.molbev.a026036
Barnosky A, Koch P, Feranec R, Wing S, Shabel A (2004) Assessing the causes of late pleistocene extinctions on the continents. Science 306:70–75. https://doi.org/10.1126/science.1101476
Benecke N (1999) The evolution of the vertebrate fauna in the Crimean Mountains from the late pleistocene to the mid-Holocene. Archäol Eurasien 6:43–57
Bilton DT, Jaarola M (1996) Isolation and purification of vertebrate DNAs. In Clapp JP (ed) Species diagnostics protocols. Methods in molecular biology 50. Humana Press, Totowa, New Jersey, pp 25–37. https://doi.org/10.1385/0-89603-323-6:25
Capelli C, Tschentscher F (2005) Protocols for ancient DNA typing. Methods Mol Biol 297:265–278. https://doi.org/10.1385/1-59259-867-6:265
Chabai VP, Stupak DV, Veselskyi AP, Dudnyk DV (2020) The cultural and chronological variability of the Epigravettian of the Middle Dnieper basin. Arheologia 2:5–31. https://doi.org/10.15407/archaeologyua2020.02.005 [In Ukrainian]
Darriba D, Taboada GL, Doallo R, Posada D (2012) jModelTest 2: more models, new heuristics and parallel computing. Nat Methods 9(8):772. https://doi.org/10.1038/nmeth.2109
Demay L, Stupak DV (2021) New complex investigations of the Novhorod-Siverskyi Upper Palaeolithic site. Arheologia 4:5–34. https://doi.org/10.15407/arheologia2021.04.005
Ding L, Chen C, Wang H, Zhang B (2016) Complete mitochondrial DNA sequence of Lepus tolai (Leporidae: Lepus). Mitochondrial DNA Part A 27(3):2085–2086. https://doi.org/10.3109/19401736.2014.982568
Edgar RC (2004) MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res 32(5):1792–1797. https://doi.org/10.1093/nar/gkh340
Ferreira MS, Jones MR, Callahan CM, Farelo L, Tolesa Z, Suchentrunk F, Boursot P, Mills LS, Alves PC, Good JM, Melo-Ferreira J (2021) The legacy of recurrent introgression during the radiation of hares. Syst Biol 70:593–607. https://doi.org/10.1093/sysbio/syaa088
Fredsted T, Wincentz T, Villesen P (2006) Introgression of mountain hare (Lepus timidus) mitochondrial DNA into wild brown hares (Lepus europaeus) in Denmark. BMC Ecol 6:17. https://doi.org/10.1186/1472-6785-6-17
Giannoulis T, Stamatis C, Tsipourlianos A, Mamuris Z (2018) Mitogenomic analysis in european brown hare (Lepus europaeus) proposes genetic and functional differentiation between the distinct lineages. Mitochondrial DNA Part A 29(3):353–360. https://doi.org/10.1080/24701394.2016.1278540
Gorobets L, Kovalchuk O, Ridush B (2023) One or two: how many species of the genus Pyrrhocorax Tunstall, 1771 (Passeriformes, Corvidae) inhabited the Crimea during the late Pleistocene? Zoodiversity 57(2):151–170. https://doi.org/10.15407/zoo2023.02.151
Gouy M, Guindon S, Gascuel O (2010) SeaView Version 4: a multiplatform graphical user interface for sequence alignment and phylogenetic tree building. Mol Biol Evol 27(2):221–224. https://doi.org/10.1093/molbev/msp259
Gromov IM (1961) Extinct Upper Quaternary rodents from the foothill part of the Crimea. Tr Komiss Izuch Chetvertich Per 17:1–189 [In Russian]
Guindon S, Dufayard JF, Lefort V, Anisimova M, Hordijk W, Gascuel O (2010) A new algorithms and methods to estimate maximum-likelihood phylogenies: assessing the performance of PhyML 3.0. Syst Biol 59(3):307–321. https://doi.org/10.1093/sysbio/syq010
Gureev AA (1964) Fauna of the USSR: mammals, vol. 3, no. 10: Lagomorpha. Nauka, Moscow–Leningrad. [In Russian]
Halanych KM, Demboski JR, van Jansen B, Klein DR, Cook JA (1999) Cytochrome b phylogeny of north american hares and jackrabbits (Lepus, Lagomorpha) and the effects of saturation in outgroup taxa. Mol Phylogenet Evol 11:213–221. https://doi.org/10.1006/mpev.1998.0581
Hall TA (1999) BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp Ser 41:95–98
Höss M, Pääbo S (1993) DNA extraction from pleistocene bones by a silica-based purification method. Nucleic Acids Res 21(16):3913–3914. https://doi.org/10.1093/nar/21.16.3913
Iakovleva L, Djindjian F (2005) New data on mammoth bone settlements of Eastern Europe in the light of the new excavations of the Gontsy site (Ukraine). Quat Int 126–128:195–207. https://doi.org/10.1016/j.quaint.2004.04.023
Iakovleva L, Djindjian F, Maschenko EN, Konik S, Moigne AM (2012) The late Upper Palaeolithic site of Gontsy (Ukraine): a reference for the reconstruction of the hunter–gatherer system based on a mammoth economy. Quat Int 255:86–93. https://doi.org/10.1016/j.quaint.2011.10.004
Jones JR, Marín-Arroyo AB, Straus LG, Richards MP (2020) Adaptability, resilience and environmental buffering in european Refugia during the late pleistocene: insights from La Riera cave (Asturias, Cantabria, Spain). Sci Rep 10(1):1217. https://doi.org/10.1038/s41598-020-57715-2
Kalmar T, Bachranti CZ, Marcsik A, Rasko I (2000) A simple and efficient method for PCR amplifiable DNA extraction from ancient bones. Nucleic Acids Res 28(12):e67. https://doi.org/10.1093/nar/28.12.e67
Kasapidis P, Suchentrunk F, Magoulas A, Kotoulas G (2005) The shaping of mitochondrial DNA phylogeographic patterns of the brown hare (Lepus europaeus) under the combined influence of late pleistocene climatic fluctuations and anthropogenic translocations. Mol Phylogenet Evol 34:55–66. https://doi.org/10.1016/j.ympev.2004.09.007
Kosintsev P (2007) Late pleistocene large mammal faunas from the urals. Quat Int 160:112–120. https://doi.org/10.1016/j.quaint.2006.09.012
Kovalchuk O, Rekovets L, Tsvelykh A, Yanenko V, Manko V, Tajkova S (2021) Living in a time of change: late Pleistocene/Holocene transitional vertebrate fauna of Grot Skeliastyi (Crimea, Ukraine). Hist Biol 33(10):2074–2084. https://doi.org/10.1080/08912963.2020.1769094
Krokhmal’ AI, Rekovets LI (2010) Localities of small mammals from the Pleistocene of Ukraine and adjacent Territories. LAT & K, Kyiv. [In Russian]
Krokhmal’ O, Rekovets L, Kovalchuk O (2021) An updated biochronology of ukrainian small mammal faunas of the past 1.8 million years based on voles (Rodentia, Arvicolidae): a review. Boreas 50:619–630. https://doi.org/10.1111/bor.12511
Krokhmal’ O, Rekovets L, Kovalchuk O (2022) Biochronological scheme of the Quaternary of the south of Eastern Europe and its substantiation based on arvicoline teeth morphometrics. Quat Int. https://doi.org/10.1016/j.quaint.2022.12.003
Lagerholm VK, Sandoval-Castellanos E, Vaniscotte A, Potapova OR, Tomek T, Bochenski ZM, Shepherd P, Barton N, Van Dyck MC, Miller R, Höglund J, Yoccoz NG, Dalén L, Stewart JR (2017) Range shifts or extinction? Ancient DNA and distribution modelling reveal past and future responses to climate warming in cold-adapted birds. Glob Chang Biol 23(4):1425–1435. https://doi.org/10.1111/gcb.13522
Leigh J, Bryant D (2015) PopART: full-feature software for haplotype network construction. Methods Ecol Evol 6:1110–1116. https://doi.org/10.1111/2041-210X.12410
Lister AM (1987) Giant deer and giant red deer from Kent’s cavern, and the status of Strongyloceros spelaeus Owen. Trans Proc Torquay Nat Hist Soc 19:189–198
Marques JP, Seixas FA, Farelo L, Callahan CM, Good JM, Montgomery WI, Reid N, Alves PC, Boursot P, Melo-Ferreira J (2020) An annotated draft genome of the mountain hare (Lepus timidus). Genome Biol Evol 12(1):3656–3662. https://doi.org/10.1093/gbe/evz273
Matthee CA, Van Vuuren BJ, Bell D, Robinson TJ (2004) A molecular supermatrix of the rabbits and hares (Leporidae) allows for the identification of five intercontinental exchanges during the Miocene. Syst Biol 53(3):433–447. https://doi.org/10.1080/10635150490445715
Meiri M, Lister AM, Stewart JR, Straus LG, Obermaier H, González Morales MR, Marín-Arroyo AB, Higham TFG, Barnes I (2013) Late glacial recolonisation and phylogeography of european red deer (Cervus elaphus L). Mol Ecol 22(18):4711–4722. https://doi.org/10.1111/mec.12420
Melo-Ferreira J, Boursot P, Suchentrunk F, Ferrand N, Alves PC (2005) Invasion from the cold past: extensive introgression of mountain hare (Lepus timidus) mitochondrial DNA into three other hare species in northern Iberia. Mol Ecol 14:2459–2464. https://doi.org/10.1111/j.1365-294X.2005.02599.x
Melo-Ferreira J, Boursot P, Randi E, Kryukov A, Suchentrunk F, Ferrand N, Alves PC (2007) The rise and fall of the mountain hare (Lepus timidus) during pleistocene glaciations: expansion and retreat with hybridization in the Iberian Peninsula. Mol Ecol 16:605–618. https://doi.org/10.1111/j.1365-294X.2006.03166.x
Melo-Ferreira J, Boursot P, Carneiro M, Esteves PJ, Farelo L, Alves PC (2012) Recurrent introgression of mitochondrial DNA among hares (Lepus spp.) revealed by species-tree inference and coalescent simulations. Syst Biol 61(3):367–381. https://doi.org/10.1093/sysbio/syr114
Michell CT, Pohjoismäki JL, Spong G, Thulin CG (2022) Mountain- and brown hare genetic polymorphisms to survey local adaptations and conservation status of the heath hare (Lepus timidus sylvaticus, Nilsson 1831). Sci Data 9(1):667. https://doi.org/10.1038/s41597-022-01794-5
Moska M, Jakubiec J, Wierzbicki H, Strzała T, Kozyra K (2016) Low genetic variability of the edible dormouse (Glis glis) in Stolowe Mountains National Park (Poland) — preliminary results. Mamm Res 61(4):409–415. https://doi.org/10.1007/s13364-016-0282-0
Nadachowski A, Lemanik A, Fontana L, Popović D, Golubiński M, Bujalska B, Baca M (2023) Ancient DNA contradicts the presence of social voles (genus Microtus, subgenus Sumeriomys) in the late pleistocene of western Europe. Diversity 15(4):538. https://doi.org/10.3390/d15040538
Pierpaoli M, Riga F, Trocchi V, Randi E (1999) Species distinction and evolutionary relationships of the italian hare (Lepus corsicanus) as described by mitochondrial DNA sequencing. Mol Ecol 8:1805–1817. https://doi.org/10.1046/j.1365-294x.1999.00766.x
Pohjoismaki JLO, Michell C, Levanen R, Smith S (2021) Hybridization with mountain hares increases the functional allelic repertoire in brown hares. Sci Rep 11:15771. https://doi.org/10.1038/s41598-021-95357-0
Poinar HN (2003) The top 10 list: criteria of authenticity for DNA from ancient and forensic samples. Int Congr Ser 1239:575–579. https://doi.org/10.1016/S0531-5131(02)00624-6
Prost S, Knapp M, Fleming J, Hufthammer AK, Kosintsev P, Stiller M, Hofreiter M (2010) Short communication: a phantom extinction? New insights into extinction dynamics of the Don-hare Lepus tanaiticus. J Evol Biol 23:2022–2029. https://doi.org/10.1111/j.1420-9101.2010.02062.x
Rabiniak E, Rekovets L, Stewart JR, Dalén L, Barton N, Strzała T, Barkaszi Z, Kovalchuk O (2023) Late pleistocene and holocene pikas (Mammalia, Lagomorpha) from Europe and the validity of Ochotona spelaea: new insights based on mtDNA analysis. Palaeontol Electron 26(1):a3. https://doi.org/10.26879/1241
Ramirez-Silva JP, Gonzalez-Cozatl FX, Vazquez-Dominguez E, Cervantes FA (2010) Phylogenetic position of mexican jackrabbits within the genus Lepus (Mammalia: Lagomorpha): a molecular perspective. Rev Mex Biodiv 81(3):721–731
Rekovets LI (1985) Small mammals of Desna-Dnieper late palaeolithic. Naukova Dumka, Kyiv. [In Russian]
Rekovets LI (1994) Small mammals of the Anthropogene of southern part of Eastern Europe. Naukova Dumka, Kyiv. [In Russian]
Rekovets LI (1995) Periglacial micromammal faunas from the late pleistocene of Ukraine. Acta Zool Cracov 38(1):129–138
Rekovets LI, Topachevsky IV (1988) Lagomorphs (Lagomorpha, Mammalia) from the late palaeolithic fauna of Mezhirich. Paleontol Sborn 25:56–60 [In Russian]
Robinson TJ, Mathee CA (2005) Phylogeny and evolutionary origins of the Leporidae: a review of cytogenetics, molecular analyses and a supermatrix analysis. Mammal Rev 35(3–4):231–247. https://doi.org/10.1111/j.1365-2907.2005.00073.x
Rohland N, Hofreiter M (2007) Ancient DNA extraction from bones and teeth. Nat Protoc 2:1756–1762. https://doi.org/10.1038/nprot.2007.247
Ronquist F, Teslenko M, van der Mark P, Ayres DL, Darling A, Hohna S, Larget B, Liu L, Suchard MA, Huelsenbeck JP (2012) MrBayes 3.2: efficient bayesian phylogenetic inference and model choice across a large model space. Syst Biol 61(3):539–542. https://doi.org/10.1093/sysbio/sys029
Sharko F, Slobodova N, Boulygina E, Cheprasov M, Gladysheva-Azgari M, Tsygankova S, Rastorguev S, Novgorodov G, Boeskorov G, Grigorieva L, Hwang WS, Tikhonov A, Nedoluzhko A (2023) Ancient DNA of the Don-hares assumes the existence of two distinct mitochondrial clades in Northeast Asia. Genes 14:700. https://doi.org/10.3390/genes14030700
Sinitsyn AA, Praslov ND, Svezhentsev YS, Sulerzhitskii LD (1997) Radiocarbon chronology for the Upper Palaeolithic in Eastern Europe. In: Sinitsyn AA, Praslov ND (eds) Radiocarbon Chronology for the Palaeolithic in Eastern Europe and Northern Asia. Problems and perspectives. IIMK RAN, St Petersburg, pp 21–66. [In Russian]
Smith S, Sandoval-Castellanos E, Lagerholm VK, Napierala H, Sablin M, Von Seth J, Fladerer FA, Germonpré M, Wojtal P, Miller R, Stewart JR, Dalén L (2017) Nonreceding hare lines: genetic continuity since the late pleistocene in european mountain hares (Lepus timidus). Biol J Linn Soc 120(4):891–908. https://doi.org/10.1093/biolinnean/blw009
Smith AT, Johnston CH, Alves PC, Häcklander K (2018) Lagomorphs: pikas, rabbits and hares of the world. Johns Hopkins University Press, Baltimore
Sommer RS (2020) Late pleistocene and Holocene History of Mammals in Europe. In: Hackländer K, Zachos F (eds) Mammals of Europe – Past, Present, and Future. Handbook of the Mammals of Europe. Springer, Cham. https://doi.org/10.1007/978-3-030-00281-7_3
Stewart JR (1999) Intraspecific variation in modern and quaternary european Lagopus. Smithson Contrib Paleobiol 89:159–168
Thulin CG, Jaarola M, Tegelstrom H (1997) The occurrence of mountain hare mitochondrial DNA in wild brown hares. Mol Ecol 6:463–467. https://doi.org/10.1046/j.1365-294X.1997.t01-1-00199.x
Thulin CG, Fang M, Averianov AO (2006) Introgression from Lepus europaeus to L. timidus in Russia revealed by mitochondrial single nucleotide polymorphisms and nuclear microsatellites. Hereditas 143:68–76. https://doi.org/10.1111/j.2006.0018-0661.01952.x
Tsvirkun OI, Shydlovskyi PS (2022) Technological and typological features of the unit 1 lithic assemblage from Mezhyrich settlement. Arkheolohiia i davnia istoriia Ukrainy 4:58–80 [In Ukrainian]
Urantowka AD, Kroczak A, Mackiewicz P (2017) The influence of molecular markers and methods on inferring the phylogenetic relationships between the representatives of the Arini (parrots, Psittaciformes), determined on the basis of their complete mitochondrial genomes. BMC Evol Biol 17:166. https://doi.org/10.1186/s12862-017-1012-1
Walsh P, Metzger D, Higuchi R (1991) Chelex 100 as a medium for simple extraction of DNA for PCR-based typing from forensic material. Biotechniques 10(4):506–513
Waltari E, Cook JA (2005) Hares on ice: phylogeography and historical demographics of Lepus arcticus, L. othus, and L. timidus (Mammalia: Lagomorpha). Mol Ecol 14(10):3005–3016. https://doi.org/10.1111/j.1365-294X.2005.02625.x
Waltari E, Demboski JR, Klein DR, Cook JA (2004) A molecular perspective on historical biogeography of the northern high latitudes. J Mammal 85(4):591–600. https://doi.org/10.1644/BER-101
Westerman M, Krajewski C, Kear BP, Meehan L, Meredith RW, Emerling CA, Springer MS (2016) Phylogenetic relationships of dasyuromorphian marsupials revisited. Zool J Linn Soc 176(3):686–701. https://doi.org/10.1111/zoj.12323
Wu C, Wu J, Bunch TD, Li Q, Wang Y, Zhang YP (2005) Molecular phylogenetics and biogeography of Lepus in Eastern Asia based on mitochondrial DNA sequences. Mol Phylogenet Evol 37(1):45–61. https://doi.org/10.1016/j.ympev.2005.05.006
Acknowledgements
The research was carried out as part of the project ‘Development of the biota in the late Cenozoic of Ukraine’ (0120U100451) funded by the National Museum of Natural History, National Academy of Sciences of Ukraine. The authors sincerely thank the editor M. Kazimírová and two anonymous reviewers for their valuable notes and suggestions. We are also thankful to P. Mackiewicz (University of Wroclaw) for providing important consultations during the early stages of the project implementation, and to D. Stupak (Institute of Archaeology, National Academy of Sciences of Ukraine) for his kind help in literature search regarding the calibrated age of materials from the studied localities.
Funding
The research of OK and ZB was supported by the National Museum of Natural History, NAS of Ukraine (No. 0120U100451).
Author information
Authors and Affiliations
Contributions
All authors contributed equally to the study. The first draft of the manuscript was written by ER, OK, and ZB. All authors read and commented the text and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflicts of interest to declare that are relevant to the content of this article.
Ethics approval
Museum specimens used in the study were treated according to the approved policies of the respective institutions.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Rabiniak, E., Rekovets, L., Kovalchuk, O. et al. Hares from the Late Pleistocene of Ukraine: a phylogenetic analysis and the status of Lepus tanaiticus (Mammalia, Lagomorpha). Biologia 79, 87–99 (2024). https://doi.org/10.1007/s11756-023-01499-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11756-023-01499-z