Abstract
Objective
Vein graft failure is a major complication following coronary artery bypass graft surgery. There is no translational model to understand the molecular mechanisms underlying vein-graft failure. We established a clinically relevant bypass graft model to investigate the underlying pathophysiological mechanisms of vein-graft failure and identify molecular targets for novel therapies.
Methods
Six female Yucatan microswine fed with high cholesterol diet underwent off-pump bypass, using superficial epigastric vein graft, which was anastomosed to an internal mammary artery and distal left anterior descending artery. Vein-graft patency was examined 10-months after bypass surgery by echocardiography, coronary angiography, and optical coherence tomography followed by euthanasia. Coronary tissues were collected for histomorphometry studies.
Results
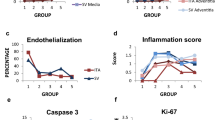
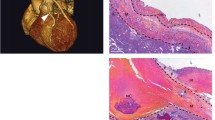
Atherosclerotic microswine were highly susceptible to sudden ventricular fibrillation with any cardiac intervention. Two out of six animals died during surgery due to ventricular fibrillation. Selection of the anesthetics and titration of their doses with careful use of inotropic drugs were the key to successful swine cardiac anesthesia. The hypotensive effects of amiodarone and the incidence of arrhythmia were avoided by the administration of magnesium sulfate. The vein-graft control tissue displayed intact endothelium with well-organized medial layer. The grafted vessels revealed complete occlusion and were covered with fibrous tissues. Expression of CD31 in the graft was irregular as the layers were not clearly defined due to fibrosis.
Conclusion
This model represents the clinical vein-graft failure and offers a novel platform to investigate the underlying molecular mechanisms of vein-graft disease and investigate novel therapeutic approaches to prevent its progression.







Similar content being viewed by others
Abbreviations
- ABGS:
-
Arterial blood gases
- ACS:
-
Activated clotted time
- CABG:
-
Coronary artery bypass graft
- CRT:
-
Capillary refill time
- EKG:
-
Electrocardiogram
- ETCO2 :
-
End tidal CO2
- HDL:
-
High density lipoprotein
- HFD:
-
High fat diet
- IMA:
-
Internal mammary artery
- LAD:
-
Left anterior descending artery
- LDL :
-
Low density lipoprotein
- OCT:
-
Optical coherence tomography
- SEV:
-
Superficial epigastric vein
- TG:
-
Triglycerides
References
Safaie N, Montazerghaem H, Jodati A, Maghamipour N. In-Hospital complications of coronary artery bypass graft surgery in patients older than 70 years. J Cardiovasc Thorac Res. 2015;7:60–2.
Velazquez EJ, Lee KL, Jones RH, Al-Khalidi HR, Hill JA, Panza JA, et al. Coronary-artery bypass surgery in patients with ischemic cardiomyopathy. N Engl J Med. 2016;374:1511–20.
ter Woorst JF, van Straten AHM, Houterman S, Soliman-Hamad MA. Sex Difference in coronary artery bypass grafting: preoperative profile and early outcome. J Cardiothorac Vasc Anesth. 2019;33:2679–84.
Dianati Maleki N, Ehteshami Afshar A, Parikh PB. Management of saphenous vein graft disease in patients with prior coronary artery bypass surgery. Curr Treat Options Cardiovasc Med [Internet]. 2019 [cited 2019 Apr 5];21. Available from: http://link.springer.com/https://doi.org/10.1007/s11936-019-0714-7
Hosono M, Murakami T, Hirai H, Sasaki Y, Suehiro S, Shibata T. The risk factor analysis for the late graft failure of radial artery graft in coronary artery bypass grafting. Ann Thorac Cardiovasc Surg. 2019;25:32–8.
Maznyczka AM, Barakat MF, Ussen B, Kaura A, Abu-Own H, Jouhra F, et al. Calculated plasma volume status and outcomes in patients undergoing coronary bypass graft surgery. Heart. 2019; heartjnl-2018-314246.
Prim DA, Zhou B, Hartstone-Rose A, Uline MJ, Shazly T, Eberth JF. A mechanical argument for the differential performance of coronary artery grafts. J Mech Behav Biomed Mater. 2016;54:93–105.
Possati G, Gaudino M, Prati F, Alessandrini F, Trani C, Glieca F, et al. Long-term results of the radial artery used for myocardial revascularization. Circulation. 2003;108:1350–4.
Thankam FG, Ayoub JG, Ahmed MMR, Siddique A, Sanchez TC, Peralta RA, et al. Association of hypoxia and mitochondrial damage associated molecular patterns in the pathogenesis of vein graft failure: a pilot study. Transl Res. 2021;229:38–52.
Chandrasekhar SK, Thankam FG, Agrawal DK, Ouseph JC. 2-Interface biology of stem cell–driven tissue engineering: concepts, concerns, and approaches. In: Sharma CP, editor. Biointegration Med Implant Mater Second Edn [Internet]. Woodhead Publishing; 2020 [cited 2019 Oct 15]. p. 19–44. Available from: http://www.sciencedirect.com/science/article/pii/B9780081026809000020
Duffield JS, Lupher M, Thannickal VJ, Wynn TA. Host responses in tissue repair and fibrosis. Annu Rev Pathol Mech Dis. 2013;8:241–76.
Thankam FG, Roesch ZK, Dilisio MF, Radwan MM, Kovilam A, Gross RM, et al. Association of inflammatory responses and ECM disorganization with HMGB1 upregulation and NLRP3 inflammasome activation in the injured rotator cuff tendon. Sci Rep. 2018;8:1–14.
Thankam FG, Boosani CS, Dilisio MF, Dietz NE, Agrawal DK. MicroRNAs associated with shoulder tendon matrisome disorganization in glenohumeral arthritis. PLoS ONE. 2016;11:e0168077.
Thankam FG, Dilisio MF, Dietz NE, Agrawal DK. TREM-1, HMGB1 and RAGE in the shoulder tendon: dual mechanisms for inflammation based on the coincidence of glenohumeral arthritis. PLoS ONE. 2016;11:e0165492.
Almeida JI, Javier JJ, Mackay E, Bautista C, Proebstle TM. First human use of cyanoacrylate adhesive for treatment of saphenous vein incompetence. J Vasc Surg Venous Lymphat Disord. 2013;1:174–80.
Cable DG, Dearani JA, Pfeifer EA, Daly RC, Schaff HV. Minimally invasive saphenous vein harvesting: endothelial integrity and early clinical results11This article has been selected for the open discussion forum on the STS Web site: http://www.sts.org/annals. Ann Thorac Surg. 1998;66: 139–43.
Coupland A, Stansby G. Glue for superficial venous ablation. Rev Vasc Med. 2014;2:98–102.
Min RJ, Almeida JI, Mclean DJ, Madsen M, Raabe R. Novel vein closure procedure using a proprietary cyanoacrylate adhesive: 30-day swine model results. Phlebol J Venous Dis. 2012;27:398–403.
Radak D, Djukic N, Neskovic M. Cyanoacrylate embolization: a novelty in the field of varicose veins surgery. Ann Vasc Surg. 2019;55:285–91.
Rojas-Pena A, Koch KL, Heitner HD, Hall CM, Bergin IL, Cook KE. Quantification of thermal spread and burst pressure after endoscopic vessel harvesting: a comparison of 2 commercially available devices. J Thorac Cardiovasc Surg. 2011;142:203–8.
Schachner T, Laufer G, Bonatti J. In vivo (animal) models of vein graft disease☆. Eur J Cardiothorac Surg [Internet]. 2006 [cited 2019 Aug 16]; 30: 451–63. https://doi.org/10.1016/j.ejcts.2006.06.015
Wang D, Tediashvili G, Pecha S, Reichenspurner H, Deuse T, Schrepfer S. Vein interposition model: a suitable model to study bypass graft patency. J Vis Exp. 2017;2017:54839.
Laflamme M, DeMey N, Bouchard D, Carrier M, Demers P, Pellerin M, et al. Management of early postoperative coronary artery bypass graft failure. Interact Cardiovasc Thorac Surg [Internet]. 2012 [cited 2019 Aug 16]; 14: 452–6. https://doi.org/10.1093/icvts/ivr127
Owens CD, Gasper WJ, Rahman AS, Conte MS. Vein graft failure. J Vasc Surg. 2015;61:203–16.
Owens CD, Rybicki FJ, Wake N, Schanzer A, Mitsouras D, Gerhard-Herman MD, et al. Early remodeling of lower extremity vein grafts: inflammation influences biomechanical adaptation. J Vasc Surg. 2008;47:1235–42.
Jiang Z, Tao M, Omalley KA, Wang D, Ozaki CK, Berceli SA. Established neointimal hyperplasia in vein grafts expands via TGF-beta-mediated progressive fibrosis. Am J Physiol Heart Circ Physiol. 2009;297:H1200-1207.
Hocum Stone L, Butterick TA, Duffy C, Swingen C, Ward HB, Kelly RF, et al. Cardiac strain in a swine model of regional hibernating myocardium: effects of CoQ10 on contractile reserve following bypass surgery. J Cardiovasc Transl Res. 2016;9:368–73.
Litten-Brown JC, Corson AM, Clarke L. Porcine models for the metabolic syndrome, digestive and bone disorders: a general overview. Animal. 2010;4:899–920.
Li S-J, Liu C-H, Chang C-W, Chu H-P, Chen K-J, Mersmann HJ, et al. Development of a dietary-induced metabolic syndrome model using miniature pigs involvement of AMPK and SIRT1. Eur J Clin Invest. 2015;45:70–80.
Turk JR, Henderson KK, Vanvickle GD, Watkins J, Laughlin MH. Arterial endothelial function in a porcine model of early stage atherosclerotic vascular disease: Minipig model of endothelial dysfunction. Int J Exp Pathol. 2005;86:335–45.
Hocum Stone LL, Swingen C, Holley C, Wright C, Chappuis E, Ward HB, et al. Magnetic resonance imaging assessment of cardiac function in a swine model of hibernating myocardium 3 months following bypass surgery. J Thorac Cardiovasc Surg. 2017;153:582–90.
Acknowledgements
The authors acknowledge the support of several individuals during the initial stages of establishing the CABG model in swine. These individuals include: Gunasekar Palanikumar MBBS, Liang Mo MD, Jubing Zhang MD, Zefu Zhang MD, Marcus Balters MD, Ashok Kujur MSc, Michael J. Moulton MD, and several veterinary technicians.
Funding
The research work of DK Agrawal is supported by research grants R01HL 128063, R01 HL144125 and R01HL147662 from the National Institutes of Health, USA. The content of this review article is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors have read the journal’s policy on disclosure of potential conflicts of interest. Author E (DKA) has received grants from the National Institutes of Health. Other authors (MMR, AS, FGT, KTK) have no relevant affiliations or financial or non-financial involvement with any organization or entity with financial or non-financial interest or conflict with the subject matter or materials discussed in the manuscript apart from those disclosed. All authors (MMR, AS, FGT, KTK, DKA) declare that they have no conflict of interest.
Ethical approval
All institutional and national guidelines for the care and use of laboratory animals were followed and approved by the appropriate institutional committees.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Supplementary file1 Video 1: OPCABG using SEV as a conduit; This approach was used in all six Yucatan microswine (MOV 18992 KB)
Supplementary file2 Video 2: Graft occlusion post-sacrifice after 10 months of CABG procedure. This is a representative of 4 Yucatan microswine (MOV 16735 KB)
Rights and permissions
About this article
Cite this article
Radwan, M.M., Siddique, A., Thankam, F.G. et al. Translational model of vein graft failure following coronary artery bypass graft in atherosclerotic microswine. Gen Thorac Cardiovasc Surg 70, 445–454 (2022). https://doi.org/10.1007/s11748-021-01725-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11748-021-01725-y