Abstract
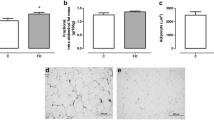
In women, bone mass undergoes changes during pregnancy and the postpartum period, which has a risk for subsequent development of osteoporosis. Thus, the present study aims to evaluate the effects of flaxseed flour in femur quality during post-weaning of dam rats. After weaning, the rats were divided into control (C, n = 7) and experimental (F, n = 7) groups treated with a diet containing 25 g of flaxseed flour in the lactating period and 15 g in the maintenance period. After 51 days post-partum, serum hormone, fatty acids composition, bone compartments, computed tomography, and biomechanical analyses were determined. Food intake, length, body mass, hormone analysis, and total bone compartments showed similar results. For biomechanical and computed tomography analysis and fatty acids composition, the F group showed higher maximum force (+12%, p < 0.05), breaking strength (+25%, p < 0.05), rigidity (+17%, p < 0.0001), and femoral head radiodensity (+15%, p < 0.05) and presented lower total polyunsaturated fatty acids (−17%, p < 0.0001) and arachidonic acid (−44%, p < 0.0001) and higher ALA (+695%, p < 0.0001) and EPA (+160%, p < 0.05). Fatty acids composition of flaxseed flour, as well as its protein profile and calcium content, were able to improve the bone quality, which may be associated with lower serum levels of arachidonic acid and higher EPA, showing an anti-inflammatory profile and increased deposition of organic matrix during the post-weaning period, and may result in prevention of future osteoporosis.


Similar content being viewed by others
Abbreviations
- AIN:
-
American Institute of Nutrition
- ALA:
-
Alpha-linolenic acid
- ARA:
-
Arachidonic acid
- B:
-
Breaking strength
- BMC:
-
Bone mineral content
- BMD:
-
Bone mineral density
- C:
-
Control
- CT:
-
Computed tomography
- DXA:
-
Dual-energy X-ray absorptiometry
- F:
-
Flaxseed flour
- HE:
-
Hematoxylin-eosin
- LNA:
-
Linoleic acid
- LV:
-
Lumbar vertebra
- N:
-
Maximum force
- MSCs:
-
Mesenchymal stem cells
- MPa:
-
Rigidity
- PGE2:
-
Series 2 prostaglandin
- PPAR-γ:
-
Receptor peroxisome proliferator-activated gamma
- SEM:
-
Standard error of the mean
References
Vadja EG, Bowman BM, Miller SC (2001) Cancellous and cortical bone mechanical properties and tissue dynamics during pregnancy, lactation, and post-lactation in the Rat. Biol Reprod 65:689–695
Kovacs CS (2014) Osteoporosis presenting in pregnancy, puerperium and lactation. Curr Opin Endocrinol Diabetes Obes 21:468–475
Bolzetta F, Veronese N, Derui M, Berton L, Carraro S, Pizzato S, Girotti G, De Ronch I, Manzato E, Coin A, Sergi G (2014) Duration of breastfeeding as a risk factor for vertebral fractures. Bone 68:41–45
Liu XS, Ardeshirpour L, VanHouten JN, Shane E, Wysolmerski JJ (2012) Site-specific changes in bone microarchitecture, mineralization, and stiffness during lactation and after weaning in mice. J Bone Miner Res 27:865–875
Yui K, Imataka G, Kawasak Y, Yamada H (2016) Increased n-3 polyunsaturated fatty acid/arachidonic acid ratios and upregulation of signaling mediator in individuals with autism spectrum disorders. Life Sci 145:205–212
Kirby BJ, Ardershirpour L, Woodrow JP, Wysolmerski JJ, Sims NA, Karaplis AC, Kovacs CS (2011) Skeletal recovery after weaning does not require PTHrP. J Bone Miner Res 26:1242–1251
Miller SC, Bowman BM (2007) Rapid inactivation and apoptosis of osteoclasts in the maternal skeleton during the bone remodeling reversal at the end of lactation. Anat Rec 290:65–73
Okyay DO, Okyay E, Dogan E, Kurtulmus S, Acet F, Taner CE (2013) Prolonged breastfeeding is an independent risk factor for postmenopausal osteoporosis. Maturitas 74:270–275
Hwang IR, Choi YK, Lee WK, Kim JG, Lee IK, Kim SW, Park KG (2016) Association between prolonged breastfeeding and bone mineral density and osteoporosis in postmenopausal women: KNHANES 2010–2011. Osteoporos Int 27:257–265
Kim JH, Kwon H, Oh SW, Lee CM, Joh HK, Kim Y, Um YJ, Ahn SH (2015) Breast feeding is associated with postmenopausal bone loss: findings from the korea national health and nutrition examination survey. Korean J Fam Med 36:216–220
Affinito P, Tommaselli GA, diCarlo C, Guida F, Nappi C (1996) Changes in bone mineral density and calcium metabolism in breastfeeding women: a one year follow-upstudy. J Clin Endocrinol Metab 81:2314–2318
Brembeck P, Lorentzon M, Ohlsson C, Winkvist A, Augustin H (2015) Changes in cortical volumetric bone mineral density and thickness, and trabecular thickness in lactating women postpartum. J Clin Endocrinol Metab 100:535–543
Jiménez-Arreola J, Aguilera-Barreiro MDLA (2015) Lactancia materna como factor preventivo para la osteoporosis em mujeres adultas. Nutr Hosp 32:2600–2605
Lenora J, Lekamwasam S, Karlsson MK (2009) Effects of multiparity and prolonged breast-feeding on maternal bone mineral density: a community-based cross-sectional study. BMC Women’s Health 9:1–6
Jiménez JAM, Moya BC, Jiménez MTM (2015) Factores nutricionales em La prevención de la osteoporosis. Nutr Hosp 32:49–55
Kettle DB (2001) Can manipulation of the ratios of essential fatty acids slow the rapid rate of postmenopausal bone loss? Altern Med Rev 6:61–77
Nakanishi A, Tsukamoto I (2015) N-3 polyunsaturated fatty acids stimulate osteoclastogenesis through PPARγ-mediated enhancement of c-Fos expression, and suppress osteoclastogenesis through PPARγ-dependent inhibition of NFkB activation. J Nutr Biochem 26:1317–1327
Farina EK, Kiel DP, Roubenoff R, Schaefer EJ, Cupples LA, Tucker KL (2011) Protective effects of fish intake and interactive effects of long-chain polyunsaturated fatty acid intakes on hip bone mineral density in older adults: the Framingham Osteoporosis Study. Am J Clin Nutr 93:1142–1151
Kruger MC, Coetzee M, Haag M, Weiler H (2010) Long-chain polyunsaturated fatty acids: selected mechanisms of action on bone. Prog Lipid Res 49:438–449
Griel AE, Kris-Etherton PM, Hilpert KF, Zhao G, West SG, Corwin RL (2007) An increase in dietary n-3 fatty acids decreases a marker of bone resorption in humans. Nutr J 6:1–8
Watkins BA, Li Y, Allen KG, Seifert MF (2000) Dietary ratio of (n26)/(n23) polyunsaturated fatty acids alters the fatty acid composition of bone compartments and biomarkers of bone formation in rats. J Nutr 130:2274–2284
Ribeiro DC, Da Silva PCA, Pereira AD, Ferolla CBB, Ribeiro CP, Duque MCA, Saldanha HS, Rozeno LP, da Costa CA, Boaventura GT (2014) Assessments of body composition and bone parameters of lactating rats treated with diet containing flaxseed meal (Linum usitatissimum) during post-weaning period. Nutr Hosp 30:366–371
Fishbeck KL, Rasmussen KM (1987) Effect of repeated cycles on maternal nutritional status, lactational performance and litter growth in ad libitum-fed and chronically food-restricted rat. J Nutr 117:1967–1975
Reeves PG (1997) Components of the AIN-93 diets as improvements in the AIN-76A diet. J Nutr 127:838–841
Lukaski HC, Hall CB, Marchello MJ, Siders WA (2001) Validation of dual x-ray absorptiometry for body-composition assessment of rats exposed to dietary stressors. Nutrition 17:607–613
Tsujio M, Mizorogi T, Kitamura I, Maeda Y, Nishijima K, Kuwahara S, Ohno T, Niida S, Nagaya M, Saito R, Tanaka S (2009) Bone mineral analysis through dual x-ray absorptiometry in laboratory animals. J Vet Med Sci 71:1493–1497
AOAC (2002) Official methods of analysis. AOAC International, Gaithersburg
Ribeiro DC, Pereira AD, Da Silva PCA, dos Santos AS, Santana FC, Boueri BF, Pessanha CR, de Abreu MD, Mancini-Filho J, da Silva EM, do Nascimento-Saba CC, da Costa CA, Boaventura GT (2015) Flaxseedflour (Linum usitatissinum) consumption improves bone quality and decreases the adipocyte área of lactating rats in the post-weaning period. Int J Food Sci Nutr 67:29–34
Costa CA, Carlos AS, Gonzalez GP, Reis RP, Ribeiro Mdos S, Dos Santos Ade S, Monteiro AM, de Moura EG, Nascimento-Saba CC (2012) Diet containing low n-6/n-3 polyunsaturated fatty acids ratio, provided by canola oil, alters body composition and boné quality in Young rats. Eur J Nutr 51:191–198
Latempa AMA, Almeida SA, Nunes NF, da Silva EM, Guimarães JG, Poskus LT (2015) Techniques for restoring enlarged canals: an evaluation of fracture resistance and bond strength. Int Endod J48:28–36
Boeyens JCA, Deepak V, Chua WH, Kruger MC, Joubert AM, Coetzee M (2014) Effects of ω3- and ω6-polyunsaturated fatty acids on RANKL-induced osteoclast differentiation of RAW264.7 cells: a comparative in vitro study. Nutrients 6:2584–2601
Hsu SC, Huang CJ (2006) Reduced fat mass in rats fed a high oleic acid-rich safflower oil diet is associated with changes in expression of hepatic PPARα and adipose SREBP-1c-regulated genes. J Nutr 136:1779–1785
Wauquier F, Léotoing L, Philippe C, Spilmont M, Coxam V, Wittrant Y (2015) Pros and cons of fatty acids in bone biology. Prog Lipid Res 58:121–145
Wallis JG, Watts JL, Browse J (2002) Polyunsaturated fatty acid synthesis: what will they think of next? Trends Biochem Sci 27:467–473
Ammann P, Rizzoli R (2003) Bone strength and its determinants. Osteoporos Int 14:13–18
Sedlin ED (1965) A archeological model for cortical bone. A study of the physical properties of human femoral samples. Acta Orthop Scand Suppl 83:1–77
Uzun G, Hersek N, Tincer T (1999) Effect of five woven fiber reinforcements on the impact and transverse strength of a denture base resin. J Prosthet Dent 81:616–620
Da Costa CA, Da Silva PCA, Ribeiro DC, Pereira AD, Dos Santos AS, de Abreu MD, Pessoa LR, Boueri BF, Pessanha CR, do Nascimento-Saba CC, da Silva EM, Boaventura GT (2016) Effects of diet containing flaxseed flour (Linum usitatissimum) up on body adiposity and bone health in young male rats. Food Funct 17:698–703
Dias CB, Wood LG, Phang M, Garg ML (2015) Kinetics of omega-3 polyunsaturated fatty acids when co-administered with saturated or omega-6 fats. Metabolism 64:1658–1666
Goyal A, Sharma V, Upadhyay N, Gill S, Sihag M (2014) Flax and flaxseed oil: an ancient medicine & modern functional food. J Food Sci Technol 51:1633–1653
Florencio-Silva R, Sasso GRS, Sasso-Cerri E, Simões MJ, Cerri PS (2015) Biology of bone tissue: structure, function, and factors that influence bone cells. Biomed Res Int. doi:10.1155/2015/421746
Lau BYY, Cohen DJA, Wendy EW, Ma DW (2013) Investigating the role of polyunsaturated fatty acids in bone development using animal models. Molecules 18:14203–14227
Wendy EW, Yuan YV, Cheung AM, Thompson LU (2001) Exposure to purifiedlignan from flaxseed (Linum usitatissimum) alters bone development in female rats. Br J Nutr 86:499–505
Acknowledgements
This project and scholarships were supported by Brazilian foundations such as the State of Rio de Janeiro Carlos Chagas Filho Research Foundation (FAPERJ), Coordination for the Enhancement of Higher Education Personnel (CAPES) and National Counsel of Technological and Scientific Development (CNPq). We are thankful to the Laboratory of Nutrition and Functional Assessment (LANUFF), College of Nutrition, Fluminense Federal University for technical assistance and use of DXA equipment. We are very grateful the students Maíra Duque, Bianca Boueri, and Carolina Pessanha for technical assistance.
Authors contribution
ADP treated the animals during experimental period and performed the computed tomography; FCS and JM-F performed the fatty acid composition; EMS performed the biomechanical analyses; CASC and GTB designed the study. All authors contributed to and approved the final manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
Financial Support
This work was supported by The State of Rio de Janeiro Carlos Chagas Filho Research Foundation (FAPERJ) (Number Process 103373/2012 and 477763/2011).
About this article
Cite this article
Ribeiro, D.C., Pereira, A.D., de Santana, F.C. et al. Incorporation of Flaxseed Flour as a Dietary Source for ALA Increases Bone Density and Strength in Post-Partum Female Rats. Lipids 52, 327–333 (2017). https://doi.org/10.1007/s11745-017-4245-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11745-017-4245-2