Abstract
Subclinical hypothyroidism (SH), defined as increased serum thyroid-stimulating hormone (TSH) with normal free T4 (fT4) levels, is frequently observed in the general population. Prevalence ranges from 0.6% to 1.8% in the adult population, depending on age, sex, and iodine intake. Several studies reported a worse prognosis in patients with heart failure with reduced ejection fraction (HFrEF) and SH, but they considered heterogeneous populations suffering mainly from severe SH. Aim of this study was to evaluate if SH was independently associated with the occurrence of cardiovascular death considering 30 months of follow-up. 277 HFrEF patients enrolled in the prospective, multicenter, observational T.O.S.CA. (Terapia Ormonale Scompenso CArdiaco) registry, were included in this analysis. Patients were divided into two groups according to the presence of SH (serum TSH levels > 4.5 mIU/L with normal fT4 levels). Data regarding clinical status, echocardiography, and survival were analyzed. Twenty-three patients displayed SH (87% mild vs 13% severe), while 254 were euthyroid. No differences were found in terms of age, sex, HF etiology, and left ventricular ejection fraction. When compared with the euthyroid group, SH patients showed higher TSH levels (7.7 ± 4.1 vs 1.6 ± 0.9, p < 0.001), as expected, with comparable levels of fT4 (1.3 ± 0.3 vs 1.3 ± 0.3, p = NS). When corrected for established predictors of poor outcome in HF, the presence of SH resulted to be an independent predictor of cardiovascular mortality (HR: 2.96; 5–95% CI:1.13–7.74; p = 0.03). Since thyroid tests are widely available and inexpensive, they should be performed in HF patients to detect subclinical disorders, evaluate replacement therapy, and improve prognosis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Chronic heart failure (CHF) is a major and growing healthcare issue with increasing prevalence worldwide, high medical costs and burdened by a still unacceptable 5-year mortality rate close to 50% [1, 2]. Despite advances due to pharmacological and non-pharmacological therapies, patients with HF often display concomitant comorbidities that elevate their level of complexity, increase patients’ frailty and, most importantly, worsen their already poor prognosis [3]. Complementing this assumption, efforts have been made in recent years in identifying clusters of patients with aggressive comorbidities who may nevertheless benefit from innovative and promising therapeutic approaches. Thyroid dysfunctions represent one of the most investigated hormonal axes in HF patients [4] and has been associated with clinically relevant outcomes, including increased cardiac-related hospitalization and mortality [5, 6]. Current guidelines recommend assessment of thyroid function in all HF patients as both hypo- and hyperthyroidism may cause or precipitate HF [7]. Additionally, new evidence suggests that subclinical hypothyroidism (SH) is linked to cardiovascular diseases and HF, as it impairs lipid profile, endothelial function, blood pressure values, coagulative homeostasis and promotes myocardial remodeling [8,9,10]. However, to date the impact of SH in the prognosis of outpatients with CHF remains poorly investigated. Moreover, most investigations support implementation of replacement therapy in this subset of patients. In this scenario, the aim of the present study was to evaluate the prognostic impact of SH in a population of well-characterized CHF patients enrolled in the T.O.S.CA. registry, a multicenter, prospective observational registry focused on hormonal abnormalities in HF [11].
Methods
Study population and study procedures
A total of 277 patients with HF with reduced ejection fraction (HFrEF) enrolled within the prospective, multicentric, observational T.O.S.CA. (Terapia Ormonale Scompenso CArdiaco) registry were included in this analysis. Study design has been previously described [11]. Briefly, the T.O.S.CA. registry enrolled 480 consecutive stable CHF patients with left ventricular ejection fraction (LVEF) ≤ 45%, on stable medications for at least 3 months before enrollment, including any beta-blocker which had to be started at least 6 months before entering the study. Exclusion criteria included recent acute decompensation or acute coronary syndrome, severe liver cirrhosis in Child–Turcotte–Pugh stage B or C, clinically relevant kidney disease (creatinine level > 2.5 mg/dl), active malignancy and current hormonal treatment or overt endocrine diseases. In this sub-analysis, we excluded patients with altered fT4, subclinical hyperthyroidism, and low T3 syndrome. A total of 277 patients were selected and divided according to the presence of SH. SH was defined as TSH levels higher than 4.5 mIU/L with normal fT4 levels and classified according to the elevation in serum TSH level: mildly increased TSH levels (4.0–10.0 mU/l), and more severely increased TSH value(> 10 mU/l) [12]. Patient demographics, blood chemistry measurements, and clinical characteristics were recorded at the time of the enrollment. Echocardiography (including two-dimensional, Doppler, Color, and tissue Doppler analysis) was also performed. Primary endpoint of the study was cardiovascular death.
The study protocol was approved by the Ethics Committees of all participating centers and all patients gave written informed consent. All procedures were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Statistical analysis
Normally distributed continuous variables were expressed as mean ± standard deviation. Categorical variables were expressed as counts and percentages. Patients were compared according to the presence of SH or normal TSH levels with unpaired Student’s t-test. Categorical variables were evaluated with χ2 test.
The association between analyzed variables and survival was evaluated using Cox proportional hazard regression analysis. Both univariate and multivariable linear models were used. For the multivariable analysis, established predictors of poor outcome in HF were employed as covariates [i.e., age, sex, body mass index, etiology, New York Heart Association (NYHA) class, and LVEF]. Kaplan–Meier curves for cumulative survival were constructed to assess the impact of SH on the primary endpoint of cardiovascular mortality. Differences in event rates between the groups were compared with the Cox–Mantel log-rank test. A P value < 0.05 was considered statistically significant.
Statistical analysis was performed using the R statistical programming environment, version 3.5.
Results
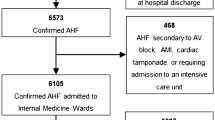
From the T.O.S.CA. registry cohort of 480 patients enrolled in 19 participating centers from April 2013 to July 2017 [13], 277 patients met the inclusion criteria and were included in the present analysis (Fig. 1). Of these, 254 (91.7%) patients were euthyroid and 23 (8.3%) patients had SH (87% mild vs 13% severe). No patient was lost to follow-up. Baseline demographic and clinical characteristics of the study population are described in Table 1. SH patients were older, had lower body mass index (BMI) and blood pressure, and higher NT-pro-BNP levels compared to non-SH patients. No differences were found with regard to NYHA class, HF etiology, heart rate, and ejection fraction between the two groups. As expected, SH patients showed higher TSH levels, with similar levels of fT4, compared to non-SH patients (7.7 ± 4.1 vs 1.6 ± 0.9, p < 0.001; 1.3 ± 0.3 vs 1.3 ± 0.3, p = NS, respectively).
At the end of the 30-month follow-up period, 131 non-SH patients (51.6%) experienced the primary endpoint of cardiovascular death compared with 13 patients (56.5%) in SH group (p < 0.01), as shown in Fig. 2.
Cox regression analysis
At univariate Cox proportional hazard regression analyses, the presence of SH was associated with cardiovascular mortality (HR 3.2 [1.3–7.9], p = 0.01) (Table 2). After correction for established predictors of poor outcome in HF employed as covariates in a multivariable Cox regression model (i.e., age, sex, body mass index, etiology, NYHA class, and LVEF), the presence of SH resulted to be an independent predictor of outcome (HR: 2.96, [1.13–7.74], p = 0.03) (Table 3).
Discussion
In the present study, we document a relationship between SH and cardiovascular mortality in HF patients with ejection fraction ≤ 45%, which is maintained even after correction with established predictors of poor outcome in HF. The novelty of our study is that this association was observed in a population: (1) consisting mainly of patients with mild SH, (2) enrolled in a prospective registry, specifically designed to investigate the role of endocrine alterations in a large cohort of well-characterized chronic HF patients, (3) after exclusion of patients with other thyroid abnormalities (i.e., low T3 syndrome, and clinical thyroid disorders), (4) with a long-term follow-up, and (5) after adjustment for the main predictors of poor outcome in HF.
The relationship between clinical thyroid disorders and cardiovascular outcomes in patients with HF has long been evaluated, indeed, ESC HF guidelines recommend the assessment of thyroid function in all patients, as both hypo- and hyperthyroidism may cause or precipitate HF [7]. In contrast, the burden of SH on the prognosis of patients with HF is still debated and, replacement therapy is usually prescribed only for TSH levels > 10 mIU/L, particularly in patients < 70 years [14, 15].
Studies reporting outcomes as a function of SH in HF have shown inconsistent results.
In the study of Frey and colleagues, SH was diagnosed in 4.6% of HF patients but was not associated with increased mortality risk, even after adjusting for age [16]. Similarly, in the CORONA trial, hypothyroidism did not result an independent predictor of adverse clinical outcomes in HF, when NT-proBNP levels were included in multivariable models [17]. Chen and collaborators studied a large HF population dividing patients into quartiles based on TSH levels: highest TSH levels were associated with increased mortality compared with patients with lower TSH levels [6]. However, the results of these two studies were hampered by the absence of fT3 and fT4 values, thus, patients with elevated TSH might have clinical hypothyroidism. Wang et al. analyzed complete thyroid function profile in 572 consecutive hospitalized HF patients with idiopathic dilated cardiomyopathy, reporting a 23% prevalence of SH which was an independent predictor of mortality. Thyroid hormone profile was assessed before patients’ discharge, when HF symptoms were controlled with oral HF medications [18]. Hayashi et al. reported SH in 58/274 patients hospitalized for acute decompensated HF. SH was associated with a significantly lower long-term survival rate compared with patients with normal thyroid function. However, increased TSH levels were detected upon admission for acute decompensated HF, thus, the authors investigated the relationship between SH and outcomes in HF in a setting of patients with instability of the hypothalamic–pituitary–thyroid axis [19]. Similarly, Sato et al. revealed an association between SH and adverse prognosis in HF patients, associated with lower peak breath-by-breath oxygen consumption and higher mean pulmonary arterial pressure. Unlike our study, their study population was enrolled during hospitalization for decompensated HF and the presence of low T3 syndrome was not considered [20]. Kannan et al. examined the prevalence of thyroid dysfunctions and its association with cardiovascular outcomes in a large cohort of HF patients. The authors showed that SH, with a TSH levels between 7.00 and 19.99 mIU/L, was associated with increased risk of the composite end point of ventricular assist device placement, heart transplantation, or death, whereas SH with TSH levels between 4.51 and 6.99 mIU/L was not associated with outcomes. In our study, the association between SH and cardiovascular death in HF was observed in a population consisting mainly of patients with mild SH. However, in the study of Kannan et al., data on clinical characteristics, HF severity, comorbid conditions, and medications in the subgroup of SH patients, that may explain the differences with our results, were not reported [5].
Several mechanisms could be responsible for the effects of SH in HF. Impairment of left ventricular systolic and diastolic function has been described in patients with SH [20,21,22,23] and a robust MRI study demonstrated that SH was associated with decreased stroke volume and cardiac output, with a complete normalization following thyroxine replacement therapy [21]. Patients with SH have shown vascular abnormalities, involving both reduced vascular compliance and impaired endothelial function through reduction of nitric oxide availability [24, 25]. Berezin et al. suggested that SH in patients with HF might be associated with an impaired release pattern of circulating extracellular microparticles with a predominance of apoptotic-derived microparticles [26]. In patients with SH, TSH levels have been directly correlated with levels of inflammatory markers, such as CRP, interleukin-6 (IL-6), and erythrocyte sedimentation rate (ESR) [27,28,29]. Moreover, SH was associated with higher levels of systolic and diastolic blood pressure, total cholesterol, LDL-c, and triglycerides, all risk factors for coronary heart disease development [30, 31]. The effect of SH on renal hemodynamics remains unclear with conflicting results in the literature [32, 33]. However, in patients with kidney failure, requiring hemodialysis, SH was associated with higher mortality compared with patients with normal thyroid function [34].
The results of the present study pave the way for implementing thyroid hormone replacement therapy in HF. Current guidelines provide general recommendations to correct SH when the TSH is > 10 mIU/L, particularly in patients < 70 years, and to consider replacement therapy at lower TSH levels (7–10 mIU/L) [7, 14, 15, 35]. Indeed, no randomized trials investigated efficacy and safety of replacement therapy in HF patients with SH [8]. However, preclinical investigations and some non-randomized controlled clinical studies have shown a beneficial effects of thyroid hormone therapy on cardiac function [36,37,38]. In our study, we mainly enrolled patients affected by mild SH (TSH between 4 and 10 mIU/L), who should not receive replacement treatment according to current guidelines. Our results strengthen the need of prospective cardiovascular outcome studies and dose–response trials to understand better define the prognostic impact of levothyroxine replacement therapy in CHF patients.
In our previous work, a significant association between low T3 syndrome and the composite endpoint of all-cause mortality and cardiovascular hospitalization has been demonstrated in HF patients [4]. In addition, the results of the present study further highlight the importance of assessing thyroid profile in patients with HF to drive therapies and improve prognosis.
Limitations
Our study presents several limitations. First, the observational character of our study is acknowledged [11]. However, our study population is well characterized, and we have assessed outcomes over a long follow-up period which justifies the relatively small sample size. Second, patients did not receive drugs currently recommended in the treatment of HF (i.e., ARNI and SGLT2i), since the study ended in July 2017. Finally, despite we tried to adjust for clinically relevant parameters, it was not possible to correct our results for all variables that may affect HF outcome.
Conclusions
The results of the present study suggest that SH is associated with adverse prognosis in HF patients, with multiple implications on clinical practice. Since thyroid tests are widely available and inexpensive, TSH, fT3, and fT4 should be assessed in all HF patients to detect early clinical and subclinical disorders, to evaluate replacement therapy and to improve prognosis. Further larger studies are needed to confirm the association between SH and HF, and to evaluate the role of thyroid hormone replacement therapy, especially in patients with mild SH.
Data availability
Not applicable.
Change history
23 July 2024
A Correction to this paper has been published: https://doi.org/10.1007/s11739-024-03717-1
References
Meta-analysis Global Group in Chronic Heart Failure (MAGGIC) (2012) The survival of patients with heart failure with preserved or reduced left ventricular ejection fraction: an individual patient data meta-analysis. Eur Heart J 33:1750–1757
Virani SS, Alonso A, Benjamin EJ, Bittencourt MS, Callaway CW, Carson AP et al (2020) Heart disease and stroke statistics-2020 update: a report from the American Heart Association. Circulation 141:e139–e596
Bozkurt B, Aguilar D, Deswal A, Dunbar SB, Francis GS, Horwich T et al (2016) Contributory risk and management of comorbidities of hypertension, obesity, diabetes mellitus, hyperlipidemia, and metabolic syndrome in chronic heart failure: a scientific statement from the American Heart Association. Circulation 134:e535–e578
Cittadini A, Salzano A, Iacoviello M, Triggiani V, Rengo G, Cacciatore F et al (2021) Multiple hormonal and metabolic deficiency syndrome predicts outcome in heart failure: the T.O.S.CA. Registry. Eur J Prev Cardiol 28:1691–1700
Kannan L, Shaw PA, Morley MP, Brandimarto J, Fang JC, Sweitzer NK et al (2018) Thyroid dysfunction in heart failure and cardiovascular outcomes. Circ Heart Fail 11:e005266
Chen S, Shauer A, Zwas DR, Lotan C, Keren A, Gotsman I (2014) The effect of thyroid function on clinical outcome in patients with heart failure. Eur J Heart Fail 16:217–226
McDonagh TA, Metra M, Adamo M, Gardner RS, Baumbach A, Böhm M et al (2023) 2023 Focused update of the 2021 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J 44:3627–3639
Triggiani V, Cittadini A, Lisco G (2022) Effect of levothyroxine replacement therapy in patients with subclinical hypothyroidism and chronic heart failure: a systematic review. Front Endocrinol (Lausanne) 13:1013641
Iacoviello M, Guida P, Guastamacchia E, Triggiani V, Forleo C, Catanzaro R et al (2008) Prognostic role of sub-clinical hypothyroidism in chronic heart failure outpatients. Curr Pharm Des 14:2686–2692
Rodondi N, Newman AB, Vittinghoff E, de Rekeneire N, Satterfield S, Harris TB et al (2005) Subclinical hypothyroidism and the risk of heart failure, other cardiovascular events, and death. Arch Intern Med 165:2460–2466
Bossone E, Arcopinto M, Iacoviello M, Triggiani V, Cacciatore F, Maiello C et al (2018) Multiple hormonal and metabolic deficiency syndrome in chronic heart failure: rationale, design, and demographic characteristics of the T.O.S.CA. Registry. Intern Emerg Med 13:661–671
Pearce SH, Brabant G, Duntas LH, Monzani F, Peeters RP, Razvi S et al (2013) 2013 ETA guideline: management of subclinical hypothyroidism. Eur Thyroid J 2:215–228
Arcopinto M, Salzano A, Giallauria F, Bossone E, Isgaard J, Marra AM et al (2017) Growth hormone deficiency is associated with worse cardiac function, physical performance, and outcome in chronic heart failure: insights from the T.O.S.CA. GHD study. PLoS ONE 12:e0170058
Peeters RP (2017) Subclinical hypothyroidism. N Engl J Med 376:2556–2565
Stott DJ, Rodondi N, Kearney PM, Ford I, Westendorp RGJ, Mooijaart SP et al (2017) Thyroid hormone therapy for older adults with subclinical hypothyroidism. N Engl J Med 376:2534–2544
Frey A, Kroiss M, Berliner D, Seifert M, Allolio B, Güder G et al (2013) Prognostic impact of subclinical thyroid dysfunction in heart failure. Int J Cardiol 168:300–305
Perez AC, Jhund PS, Stott DJ, Gullestad L, Cleland JG, van Veldhuisen DJ et al (2014) Thyroid-stimulating hormone and clinical outcomes: the CORONA trial (controlled rosuvastatin multinational study in heart failure). JACC Heart Fail 2:35–40
Wang W, Guan H, Gerdes AM, Iervasi G, Yang Y, Tang YD (2015) Thyroid status, cardiac function, and mortality in patients with idiopathic dilated cardiomyopathy. J Clin Endocrinol Metab 100:3210–3218
Hayashi T, Hasegawa T, Kanzaki H, Funada A, Amaki M, Takahama H et al (2016) Subclinical hypothyroidism is an independent predictor of adverse cardiovascular outcomes in patients with acute decompensated heart failure. ESC Heart Fail 3:168–176
Sato Y, Yoshihisa A, Kimishima Y, Kiko T, Watanabe S, Kanno Y et al (2018) Subclinical hypothyroidism is associated with adverse prognosis in heart failure patients. Can J Cardiol 34:80–87
Ripoli A, Pingitore A, Favilli B, Bottoni A, Turchi S, Osman NF et al (2005) Does subclinical hypothyroidism affect cardiac pump performance? Evidence from a magnetic resonance imaging study. J Am Coll Cardiol 45:439–445
Di Bello V, Monzani F, Giorgi D, Bertini A, Caraccio N, Valenti G et al (2000) Ultrasonic myocardial textural analysis in subclinical hypothyroidism. J Am Soc Echocardiogr 13:832–840
Biondi B, Cappola AR, Cooper DS (2019) Subclinical hypothyroidism: a review. JAMA 322:153–160
Asoğlu E, Akbulut T, Doğan Z, Asoğlu R (2021) Evaluation of the aortic velocity propagation, epicardial fat thickness, and carotid intima-media thickness in patients with subclinical hypothyroidism. Rev Cardiovasc Med 22:959–966
Owen PJ, Sabit R, Lazarus JH (2007) Thyroid disease and vascular function. Thyroid 17:519–524
Berezin AE, Kremzer AA, Martovitskaya YV, Samura TA, Berezina TA (2016) Pattern of circulating endothelial-derived microparticles among chronic heart failure patients with dysmetabolic comorbidities: the impact of subclinical hypothyroidism. Diabetes Metab Syndr 10:29–36
Taddei S, Caraccio N, Virdis A, Dardano A, Versari D, Ghiadoni L et al (2006) Low-grade systemic inflammation causes endothelial dysfunction in patients with Hashimoto’s thyroiditis. J Clin Endocrinol Metab 91:5076–5082
Vayá A, Giménez C, Sarnago A, Alba A, Rubio O, Hernández-Mijares A et al (2014) Subclinical hypothyroidism and cardiovascular risk. Clin Hemorheol Microcirc 58:1–7
Gupta G, Sharma P, Kumar P, Itagappa M (2015) Study on subclinical hypothyroidism and its association with various inflammatory markers. J Clin Diagn Res 9:BC04-6
Razvi S, Shakoor A, Vanderpump M, Weaver JU, Pearce SH (2008) The influence of age on the relationship between subclinical hypothyroidism and ischemic heart disease: a metaanalysis. J Clin Endocrinol Metab 93:2998–3007
Cai P, Peng Y, Chen Y, Wang Y, Wang X (2021) Blood pressure characteristics of subclinical hypothyroidism: an observation study combined with office blood pressure and 24-h ambulatory blood pressure. J Hypertens 39:453–460
Tsuda S, Nakayama M, Matsukuma Y, Yoshitomi R, Haruyama N, Fukui A et al (2021) Subclinical hypothyroidism is independently associated with poor renal outcomes in patients with chronic kidney disease. Endocrine 73:141–150
Meuwese CL, van Diepen M, Cappola AR, Sarnak MJ, Shlipak MG, Bauer DC et al (2019) Low thyroid function is not associated with an accelerated deterioration in renal function. Nephrol Dial Transplant 34:650–659
Rhee CM, Kim S, Gillen DL, Oztan T, Wang J, Mehrotra R et al (2015) Association of thyroid functional disease with mortality in a national cohort of incident hemodialysis patients. J Clin Endocrinol Metab 100:1386–1395
Feller M, Snel M, Moutzouri E, Bauer DC, de Montmollin M, Aujesky D et al (2018) Association of thyroid hormone therapy with quality of life and thyroid-related symptoms in patients with subclinical hypothyroidism: a systematic review and meta-analysis. JAMA 320:1349–1359
Khalife WI, Tang YD, Kuzman JA, Thomas TA, Anderson BE, Said S et al (2005) Treatment of subclinical hypothyroidism reverses ischemia and prevents myocyte loss and progressive LV dysfunction in hamsters with dilated cardiomyopathy. Am J Physiol Heart Circ Physiol 289:H2409–H2415
Gerdes AM, Iervasi G (2010) Thyroid replacement therapy and heart failure. Circulation 122:385–393
Zhang M, Sara JD, Matsuzawa Y, Gharib H, Bell MR, Gulati R et al (2016) Clinical outcomes of patients with hypothyroidism undergoing percutaneous coronary intervention. Eur Heart J 37:2055–2065
Funding
Open access funding provided by Università degli Studi di Napoli Federico II within the CRUI-CARE Agreement. This work was partially supported by funding of the Italian Ministry of Health [Ricerca corrente].
Author information
Authors and Affiliations
Consortia
Contributions
MDL, RDA, and AC contributed to the study conception and design. Material preparation, data collection, and analysis were performed by MDL and RDA. The first draft of the manuscript was written by MDL, RDA, and AC. MDL, RDA and AC interpreted results and commented on the manuscript. All the listed authors contributed to patients’ enrollment and clinical management. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declared no potential conflict of interest with respect to the research, authorship, and publication of this paper.
Human and animal rights statement and Informed consent
Human and animal rights and Informed consent: Each patient participated on a voluntary way and provided an individual infirmed consent.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
“The original online version of this article was revised:” In this article one statement was missing under the funding section. It has been corrected.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
De Luca, M., D’Assante, R., Iacoviello, M. et al. Subclinical hypothyroidism predicts outcome in heart failure: insights from the T.O.S.CA. registry. Intern Emerg Med (2024). https://doi.org/10.1007/s11739-024-03665-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11739-024-03665-w