Abstract
Evidence supporting the effectiveness of Antimicrobial Stewardship (AMS) Programs in the emergency department (ED) setting is limited. We conducted a prospective cohort study to assess the efficacy of an AMS program in an ED and a short-stay observation unit. The intervention included periodic prospective audits (twice a week), conducted by four infectious disease consultants. Primary outcomes included the difference in the hospital mortality rate, antibiotic consumption, and the incidence of bloodstream infections (BSI) caused by multidrug resistant (MDR) bacteria, before March 2020–February 2021 and after March 2021–February 2022 when the program was implemented. Interrupted time-series analysis was performed to assess the effect of our program. During the 12-month program, we performed 152 audits and evaluated 366 antibiotic therapies out of a total of 853 patients admitted. In the intervention period, we observed a non-statistically significant decrease in total antibiotic consumption, with a change in level of − 31.2 defined daily dose/100 patient-days (PD) (p = 0.71). Likewise, we found no significant variations in the rate of BSI due to MDR Gram-positive (CT − 0.02 events/PD, p = 0.84), MDR Gram-negative bacteria (CT 0.08, p = 0.71), or Candida spp. (CT 0.008, p = 0.86). Conversely, we found a significant decrease in the mortality rate between the pre- and post-intervention periods (− 1.98 deaths/100 PD, CI − 3.9 to − 0.007, p = 0.049). The Antibiotic Stewardship Program in the ED was associated with a significant decrease in the mortality rate. More high-quality studies are needed to determine the most effective ASP strategies in this unique setting.
Similar content being viewed by others
Introduction
Antimicrobial resistance (AMR) is one of the main global health threats of the twenty-first century, significantly increasing the overall hospital mortality rate, the need for intensive care units (ICUs), the length of stay, and the healthcare costs [1].
A recently published predictive statistical model by Murray et al. [2], providing the first assessment of the global burden of AMR, estimated 4.95 million (3.62–6.57) deaths globally attributable to bacterial AMR in 2019. The six leading pathogens associated with antibiotic resistance were Escherichia coli, followed by Staphylococcus aureus, Klebsiella pneumoniae, Streptococcus pneumoniae, Acinetobacter baumannii, and Pseudomonas aeruginosa [2]. Moreover, it is currently estimated that, if no appropriate measures are taken, AMR will cost approximately 10 million lives and 10 trillion US dollars per year by 2050 [3].
To tackle the emergence of resistance, many regulatory authorities and scientific societies, such as the World Health Organization (WHO) and the European Commission and the Center for Disease Control and Prevention (ECDC), have endorsed guidelines that recommend the implementation of “Antibiotic Stewardship Programs” (ASP), to improve the appropriateness of antibiotic prescriptions, including the indication, the choice of molecules, the route of administration, and the duration of therapy [1, 4, 5].
Several studies have demonstrated a clear benefit from the implementation of stewardship programs on antibiotic consumption, selection of resistance, hospital mortality, and costs [6,7,8,9,10]. However, emergency departments (EDs) are a very particular setting, since they represent the interface between the community and hospitals, serving as a gateway of entry into the hospital [11]. Moreover, they are often overcrowded, with a high turnover of patients, the decision-making process is rapid, and antimicrobial prescriptions are usually empiric due to the lack of microbiological results [12, 13]. However, there is still a lack of literature regarding ASPs in the EDs [13, 14].
The aim of the present study was to assess the impact of a non-restrictive educational Antimicrobial Stewardship Program on the appropriateness of antimicrobial prescription in an ED of a secondary-level hospital in the Campania Region in southern Italy.
Methods
Study design and setting
We conducted a prospective cohort study with an interrupted time-series analysis in an ED and a short-stay observation unit of a secondary-level non-teaching hospital in Pozzuoli (Santa Maria delle Grazie Hospital), in the Campania Region in southern Italy. Santa Maria delle Grazie Hospital is located in Pozzuoli, about 20 km from the AOU L. Vanvitelli in Naples. The hospital includes six beds in the emergency department and ten beds in the short-stay observation unit. The hospital does not have an Infectious Disease Unit or an ID consultant, and has not yet implemented an Infection Prevention and Control (IPC) program. The study period was from March 2020 to March 2022 and was designed in accordance with the guidelines of the ORION statement [15].
Antimicrobial Stewardship Program
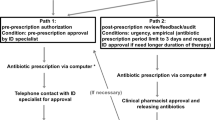
In March 2021, the Azienda Ospedaliera Universitaria of the University of Campania Luigi Vanvitelli started a 1-year Antimicrobial Stewardship Program in the ED of the Hospital Santa Maria delle Grazie of Pozzuoli, to improve the appropriateness of antibiotic prescription. Before the intervention, there were no restriction rules on antibiotic prescriptions.
We identified a multidisciplinary team, including four ID consultants, ED doctors, microbiologists, pharmacists, a statistician, and the Health Department director. ID consultants performed audits twice a week at regular intervals, every Monday and Thursday. During the audits, they evaluated all patients on antibiotic treatment, giving recommendations on indication, spectrum of action, choice of molecules, route of administration, and length of antibiotic therapies. Moreover, they were involved in writing and sharing protocols, i.e., for adequate collection of blood cultures and other microbiological samples and on empirical treatment of urinary tract infections, based on national and international recommendations. Lastly, during the audits, they evaluated the rate of adherence to these protocols.
Microbiologists provided updated reports on the susceptibility profiles of specific pathogens; pharmacists provided reports on antibiotic consumption and the related costs of the specific unit. Lastly, the statistician provided information on in-hospital mortality and length of hospital stay.
The pre-intervention phase included the period from March 2020 to February 2021, while the intervention period included the months from March 2021 to February 2022. Type and severity of infections, defined according to the Sepsis-3 definitions [16], as well as causative agents were collected for all patients who were admitted to the emergency department and emergency medicine unit during the two periods and received antimicrobial treatment.
Outcomes
We defined primary and secondary outcomes. Primary outcomes included the in-hospital mortality rate expressed as deaths/100 PD, the difference in antibiotic consumption, expressed as defined daily dose (DDD) per 100 patient-days (PD) according to WHO definitions [17], and the frequency of bloodstream infections (BSI) caused by multidrug resistant organisms (MDROs), expressed as events/100 PD, before and after the implementation of the program. The MDR categorization was applied according to the European Centre for Disease Control (ECDC) definitions [18]. Secondary outcomes included the mean length of hospital stay and antibiotic costs, expressed as euros/100 PD. Finally, we compared the mean diagnosis-related group (DRG) weight, as a parameter of clinical complexity of patients between the two periods. All variables were collected and analyzed monthly. The outcomes recorded in the intervention period were compared with those recorded in the pre-intervention period.
Statistical analysis
Continuous variables were reported as mean and standard deviation (SD) if normally distributed or as median and interquartile range (IQR) if not normally distributed, and categorical variables as absolute and relative frequencies. For continuous variables, differences were evaluated by the Student's t test; categorical variables were compared using the Chi-squared test, using exact procedures if needed. Interrupted time-series analysis (ITSA) [19] was performed to assess the effect of our AS program on mortality, DDDs, costs, and on the frequency of MDRO bloodstream infections. Differences were considered statistically significant at p < 0.05 (two-tailed tests). Analyses were performed by SPSS 23.0 (IBM, Armonk, NY, USA).
Ethics approval
The study was approved by the Ethics Committee of the University of Campania Luigi Vanvitelli, Naples (n°17539i/2022). All procedures performed in this study were in accordance with the ethics standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethics standards.
Results
Audits and characteristics of patients
During the 12-month ASP, we performed 152 audits and evaluated 366 antibiotic therapies out of a total of 853 patients admitted. The type and the severity of infections in patients admitted to the emergency department during the pre- and post-intervention phase are displayed in Supplementary Table 1. The prevalence of the different type of infections was similar between the two periods, with the exception of urinary tract infections, that were more common in the post-intervention phase (23.9 vs. 30.1%, p = 0.04); regarding the severity of patients, a higher rate of subjects with sepsis were admitted during the post-intervention phase (26.9 vs. 37.4%, p < 0.001). The distribution of microorganisms isolated from blood cultures, urine cultures, and respiratory samples was comparable during the two periods, as shown in Supplementary Table 2.
The antimicrobial treatment was deemed appropriate in 60.2% of cases during the first trimester of intervention and in 70.8% during the last trimester.
Hospital mortality
The variation in hospital mortality is shown in Table 1. In the intervention period, from March 2021 to February 2022, we found a significant decrease in the mortality rate compared to the pre-intervention period (− 1.98 deaths/100 PD, CI − 3.9 to − 0.007, p = 0.049).
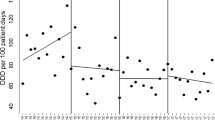
Antibiotic consumption
The variations in antibiotic consumption are shown in Table 2. We observed a non-statistically significant reduction in total antibiotic consumption during the two periods, with a change in level (CL) of − 31.2 DDD/100 PD (95% CI − 207.4 to 145.1, p = 0.71) (Table 2); in particular, a non-significant decrease was observed in piperacillin/tazobactam (change in trend, CT − 0.7, CI − 4.0 to 2.7 p = 0.67) and fluoroquinolones (CT − 1.2, CI − 4.6 to 2.3, p = 0.49). Moreover, we observed non-statistically significant variations in the use of carbapenems (CT 0.9, CI − 1.02 to 2.76, p = 0.35), third- and fourth-generation cephalosporins (CT 3.7, CI − 4.5 to 12.0, p = 0.36), and inhibitor-protected aminopenicillins (CT 3.28, − 3.7 to 10.3, p = 0.34).
Bloodstream infection rate
The effect of the AMS program on the rate of BSI due to MDR pathogens is presented in Table 3. We found no significant variations in the rate of bloodstream infections (BSI) due to MDR Gram-positive (CT − 0.02, CI − 0.2 to 0.16, p = 0.84) or MDR Gram-negative bacteria (CT 0.08, CI − 0.37 to 0.53, p = 0.71), or Candida spp. (CT 0.008, CI − 0.08 to 0.1 p = 0.86), as well as in the overall incidence rate of BSI due to Gram-positive (CT: − 0.56, CI − 1.3 to 0.18, p = 0.13) and Gram-negative bacteria (CT − 0.02, CI − 0.87 to 0.83, p = 0.96).
Secondary outcomes: mean length of hospital stay, antibiotic costs, and mean DRG weight
No significant difference was observed in the mean length of hospital stay (CL: 1.3 days, CI − 2.7 to 5.4, p = 0.49) during the study periods (Table 1). Regarding the costs, we observed a non-significant change in trend (35.6 euros/100PD/month, CI − 165.6 to 263.9, p = 0.71), with a significant increase in level (1580, CI 91.3 to 3070.5, p = 0.039) (Table 2). Finally, similar mean DRG weights were found between the two periods (2.4 ± 0.4 vs. 2.5 ± 0.3, p = 0.71).
Discussion
In the present study, we reported the effects of the implementation of an ASP in an emergency department and a short-stay observation unit of a secondary-level hospital in the Campania region. We performed 152 audits and evaluated 366 antibiotic therapies: we observed a significant decrease in the mortality rate, while a non-statistically significant variation in other primary outcomes, i.e., total antimicrobial consumption and incidence of bloodstream infections due to MDROs, as well as secondary outcomes, i.e., antibiotic expense and length of stay.
The reduction in hospital mortality, despite the higher rate of patients presenting with sepsis in the post-intervention phase, is an encouraging result, and in our opinion was mainly driven by the increase in the appropriateness of antimicrobial prescribing during the intervention period. As stated, protocols of empirical antimicrobial therapy based on local resistance rates and in accordance with international guidelines were compiled and shared with the colleagues of the emergency department. An indirect demonstration comes from the data on antibiotic appropriateness: although data on the appropriateness of antimicrobial prescriptions are unavailable for the pre-intervention period, an increase in rates of treatments deemed appropriate was registered from the first to the last trimester of intervention. Our results are consistent with several data present in the literature. A meta-analysis [20] including 37 observational studies assessing the impact of the adherence to guidelines on mortality demonstrated that the group of patients receiving antimicrobial treatment according to guidelines presented a significantly lower risk of death (RR 0.65, 95% CI 0.54–0.80, p < 0.0001). Focusing our attention on the emergency department, a retrospective study with propensity-matched analysis demonstrated that receiving an antibiotic prescription discordant with the guidelines was independently associated with 30-day mortality (hazard ratio 1.43, 95% CI 1.05–1.93) among a cohort of 630 patients admitted to the ED for severe pneumonia [21]. Moreover, many studies have demonstrated that bedside ID consultations can reduce mortality in specific clinical conditions, including S. aureus [22,23,24], Enterococcus spp. [25], and Candida spp. bloodstream infections [26]. A retrospective multicenter study enrolling 36,868 patients with S. aureus BSI from 2003 to 2014 demonstrated that subjects receiving an ID consultation presented a significant reduction in 30-day mortality [22]. Similar results were observed in a retrospective cohort study conducted among 1,691 US patients with Candida bloodstream infection [26].
The results regarding other primary outcomes are slightly in contrast with several studies published in the literature, which have demonstrated a clear benefit from the implementation of stewardship programs on antibiotic consumption and costs, and selection of resistance, in other settings [7, 9]. Several systematic reviews and meta-analyses have demonstrated significant decreases in antimicrobial consumption and costs, especially in the critical care setting [9, 27]. A network meta-analysis [28], including 42 publications, demonstrated that ASP can lead to a statistically significant reduction in the incidence of infections or colonization by MDR Gram-negative bacteria in adults admitted to ICUs. However, EDs are very particular, since they are where antibiotics are first prescribed [12, 29, 30]. Moreover, EDs are often overcrowded, with a high turnover of patients, the decision-making process is rapid, and antimicrobial prescriptions are usually empiric due to the lack of microbiological results [12, 13].
As early as 2013, May et al. shifted the attention to EDs, underscoring the need for Antimicrobial Stewardship Programs in these difficult settings [14]. We should point out that several peculiar aspects differentiate the Antimicrobial Stewardship approach in EDs compared to other in-hospital settings, as stated by May et al. [12]. First, the AS approach in EDs should focus on the clinical diagnosis, also through rapid diagnostic methods. Second, the AS should be centered on empirical therapy, focusing on the spectrum, route of administration, timing, and dosing interval. The start of an empirical therapy has a major impact on the next step since therapy is often continued by colleagues in both in-hospital and outpatient settings. In addition, the AS should emphasize the importance of collecting microbiological samples before starting antibiotic therapy, and following up patients who are discharged from hospital with an empirical treatment in an outpatient regimen. The latter point is, perhaps, the most challenging element discussed so far. Consequently, data obtained in other areas cannot be applied to the ED, and efficacy of ASP intervention in emergency departments is less clearly demonstrated compared to other hospital settings. A systematic review [31], including 43 studies on the clinical effect of ASPs in the ED, described favorable clinical outcomes in a limited number of studies; the most frequently reported benefits included an improvement in the delivery of care or a decrease in antibiotic prescription. Moreover, we should consider that most studies were judged as having an unclear or high risk of bias. Indeed, the methodology to evaluate the efficacy of ASP intervention in this setting is not well standardized. A systematic review [30], including 26 studies on Antimicrobial Stewardship Programs in EDs, described the use of heterogeneous indicators to monitor antimicrobial consumption, making it difficult to compare the results of the studies included.
Thus, while there is a growing body of literature showing that multifaceted interventions, the draft of clinical guidelines, and behavioral approaches on prescribers have an impact on antibiotic prescription in EDs, there is still a lack of high-quality studies. A prospective before–after study, conducted by Borde et al. in a non-trauma ED in Germany in 2015, aimed at reducing the use of third-generation cephalosporins and fluoroquinolones, since these antibiotics were associated with a high risk of selection of bacterial resistance [32]. The colleagues put in place a non-restrictive ASP based on guideline revision, education, intensified ID consultations and feedback, and described a significant decrease in cephalosporin use, but not in fluoroquinolone use nor overall antibiotic consumption [32]. A quasi-experimental prospective study published in 2020 [13] aimed at evaluating the impact of an ASP on antibiotic use and costs in an ED of a German hospital. The intervention included four phases that were articulated as follows: phase 1—collection of prospective epidemiological and clinical data, phase 2—development and dissemination of guidelines on empirical treatment, phase 3—prospective audit and feedback and an active infectious disease consultation service, and phase 4—random audit and periodical feedback. In the 4-year project, colleagues evaluated 42,886 patients. They reported a non-significant decrease in overall antibiotic use during phase 2 (CL − 31.12, p 0.861) and 3 (CL − 7.2, p 0.983). Moreover, they described a significant decrease in the mean yearly antibiotic costs from 691.5 euros/100 PD to 263 euros/100 PD in phase 4 (p < 0.001) and in the length of stay. The rate of Clostridioides difficile infections (CDI) decreased as well, especially during phase 2. The implementation of an ASP was not accompanied by an increase in the mortality rate, which remained stable over the whole study period (3.3% in phase 1 to 2.1% in phase 4, p 0.094).
An interesting explanation of our non-significant results is that colleagues working in the ED in Santa Maria delle Grazie Hospital were highly trained and updated on antimicrobials. In almost 65% of cases, antibiotic prescriptions were appropriate, compared to an extremely high frequency of inappropriate antimicrobial prescriptions in the EDs described in the literature, about 40–60% [12].
Our study has some limitations; in particular, the intervention was performed in only one ward, and a limited number of audits were conducted. Furthermore, we cannot be sure that the clinical characteristics of the subjects evaluated during the pre- and post-intervention periods were comparable, considering that the study was conducted in different phases of the COVID-19 pandemics. However, we observed similar types and etiologies of infections among the study periods, with a higher rate of patients presenting with sepsis during the post-intervention phase; moreover, the lack of a significant variation in the mean DRG weight among the study periods demonstrated a similar case mix of patients admitted, which strengthens the findings of our study.
In conclusion, antibiotics are frequently prescribed in EDs and short-stay observation units, and almost half of them are unnecessary or inappropriate [29, 33]. Since antimicrobial resistance is riding high at a global level, Antibiotic Stewardship Programs are needed in the emergency departments and should be tailored according to each setting. More high-level studies are needed to determine the most effective ASP strategies in this unique setting.
Data availability
Data can be requested to the corresponding author, N.C. (nicola.coppola@unicampania.it).
References
WHO. World Health Organization. Global action plan on antimicrobial resistance. World Health Organization. 2015. https://www.who.int/publications/i/item/9789241509763. Accessed 01 Mar 2023
Murray CJ, Ikuta KS, Sharara F et al (2022) Global burden of bacterial antimicrobial resistance in 2019: a systematic analysis. Lancet. https://doi.org/10.1016/S0140-6736(21)02724-0
O’Neill J. Tackling drug-resistant infections globally: final report and recommendations: the review on antimicrobial resistance; 2016. https://amr-review.org/Publications.html. Accessed 01 Mar 2023
May L, Yadav K, Gaona SD, et al. MITIGATE antimicrobial stewardship toolkit: a guide for practical implementation in adult and pediatric emergency department and urgent care settings. https://stacks.cdc.gov/view/cdc/80653. Accessed 01 Mar 2023
European Commission. A European One Health Action Plan against Antimicrobial Resistance (AMR). https://Ec.Europa.Eu/Health/Antimicrobial-Resistance/Eu-Action-on-Antimicrobial-Resistance_Es. 2017. Accessed 01 Mar 2023
Onorato L, Macera M, Calò F et al (2020) The effect of an antimicrobial stewardship programme in two intensive care units of a teaching hospital: an interrupted time series analysis. Clin Microbiol Infect. https://doi.org/10.1016/j.cmi.2019.10.021
Davey P, Marwick CA, Scott CL et al (2017) Interventions to improve antibiotic prescribing practices for hospital inpatients. Cochrane Database Syst Rev. https://doi.org/10.1002/14651858.CD003543.pub4
Karanika S, Paudel S, Grigoras C, Kalbasi A, Mylonakis E (2016) Systematic review and meta-analysis of clinical and economic outcomes from the implementation of hospital-based antimicrobial stewardship programs. Antimicrob Agents Chemother. https://doi.org/10.1128/AAC.00825-16
Baur D, Gladstone BP, Burkert F et al (2017) Effect of antibiotic stewardship on the incidence of infection and colonisation with antibiotic-resistant bacteria and Clostridium difficile infection: a systematic review and meta-analysis. Lancet Infect Dis. https://doi.org/10.1016/S1473-3099(17)30325-0
Feazel LM, Malhotra A, Perencevich EN, Kaboli P, Diekema DJ, Schweizer ML (2014) Effect of antibiotic stewardship programmes on Clostridium difficile incidence: a systematic review and meta-analysis. J Antimicrob Chemother. https://doi.org/10.1093/jac/dku046
Pulia M, Redwood R, May L (2018) Antimicrobial Stewardship in the Emergency Department. Emerg Med Clin North Am. https://doi.org/10.1016/j.emc.2018.06.012
May L, Martín Quirós A, Ten Oever J, Hoogerwerf J, Schoffelen T, Schouten J (2021) Antimicrobial stewardship in the emergency department: characteristics and evidence for effectiveness of interventions. Clin Microbiol Infect. https://doi.org/10.1016/j.cmi.2020.10.028
Savoldi A, Foschi F, Kreth F et al (2020) Impact of implementing a non-restrictive antibiotic stewardship program in an emergency department: a four-year quasi-experimental prospective study. Sci Rep. https://doi.org/10.1038/s41598-020-65222-7
May L, Cosgrove S, L’Archeveque M et al (2013) A call to action for antimicrobial stewardship in the emergency department: approaches and strategies. Ann Emerg Med. https://doi.org/10.1016/j.annemergmed.2012.09.002
Stone SP, Cooper BS, Kibbler CC et al (2007) The ORION statement: guidelines for transparent reporting of outbreak reports and intervention studies of nosocomial infection. Lancet Infect Dis. https://doi.org/10.1016/S1473-3099(07)70082-8
Singer M, Deutschman CS, Seymour C et al (2016) The third international consensus definitions for sepsis and septic shock (sepsis-3). JAMA. https://doi.org/10.1001/jama.2016.0287
WHO Collaborating Centre for Drug Statistic Methodology. WHO ATC/DDD Index 2023. https://www.whocc.no/atc_ddd_index/. Accessed 01 Mar 2023
Magiorakos AP, Srinivasan A, Carey RB et al (2012) Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect. https://doi.org/10.1111/j.1469-0691.2011.03570.x
Effective Practice and Organisation of Care (EPOC). Interrupted time series (ITS) analyses. EPOC Resources for review authors. Oslo Nor Knowl Cent Heal Serv. 2017;13(April)
Schuts EC, Hulscher MEJL, Mouton JW et al (2016) Current evidence on hospital antimicrobial stewardship objectives: a systematic review and meta-analysis. Lancet Infect Dis. https://doi.org/10.1016/S1473-3099(16)00065-7
Kang SH, Jo YH, Lee JH, Jang DH, Kim YJ, Park I (2021) Antibiotic prescription consistent with guidelines in emergency department is associated with 30-day survival in severe community-acquired pneumonia. BMC Emerg Med. https://doi.org/10.1186/s12873-021-00505-4
Goto M, Schweizer ML, Vaughan-Sarrazin MS et al (2017) Association of Evidence-based care processes with mortality in Staphylococcus aureus Bacteremia at Veterans Health Administration Hospitals, 2003–2014. JAMA Intern Med 177(10):1489–1497. https://doi.org/10.1001/jamainternmed.2017.3958
Turner RB, Valcarlos E, Won R, Chang E, Schwartz J (2016) Impact of infectious diseases consultation on clinical outcomes of patients with Staphylococcus aureus bacteremia in a community health system. Antimicrob Agents Chemother. https://doi.org/10.1128/AAC.00439-16
Goto M, Jones MP, Schweizer ML et al (2020) Association of infectious diseases consultation with long-term postdischarge outcomes among patients with Staphylococcus aureus Bacteremia. JAMA Netw Open. https://doi.org/10.1001/jamanetworkopen.2019.21048
Lee RA, Vo DT, Zurko JC, Griffin RL, Rodriguez JM, Camins BC (2020) Infectious diseases consultation is associated with decreased mortality in enterococcal bloodstream infections. Open Forum Infect Dis. https://doi.org/10.1093/ofid/ofaa064
Mejia-Chew C, O’Halloran JA, Olsen MA et al (2019) Effect of infectious disease consultation on mortality and treatment of patients with candida bloodstream infections: a retrospective, cohort study. Lancet Infect Dis. https://doi.org/10.1016/S1473-3099(19)30405-0
Karanika S, Grigoras C, Flokas ME et al (2017) The attributable burden of clostridium difficile infection to long-term care facilities stay: a clinical study. J Am Geriatr Soc 65(8):1733–1740. https://doi.org/10.1111/jgs.14863
Teerawattanapong N, Kengkla K, Dilokthornsakul P, Saokaew S, Apisarnthanarak A, Chaiyakunapruk N (2017) Prevention and control of multidrug-resistant Gram-negative bacteria in adult intensive care units: a systematic review and network meta-analysis. Clin Infect Dis. https://doi.org/10.1093/cid/cix112
Pulcini C (2015) Antimicrobial stewardship in emergency departments: a neglected topic. Emerg Med J. https://doi.org/10.1136/emermed-2014-204220
Ruiz-Ramos J, Alcón EV, Ramos FM, Santolaya-Perrín R, Tey JMG (2021) Antimicrobial stewardship programs in emergency departments: how do we measure antimicrobial use? A systematic review. Rev Esp Quimioter. https://doi.org/10.37201/req/028.2021
Losier M, Ramsey TD, Wilby KJ, Black EK (2017) A systematic review of antimicrobial stewardship interventions in the emergency department. Ann Pharmacother. https://doi.org/10.1177/1060028017709820
Borde JP, Kern WV, Hug M et al (2015) Implementation of an intensified antibiotic stewardship programme targeting third-generation cephalosporin and fluoroquinolone use in an emergency medicine department. Emerg Med J. https://doi.org/10.1136/emermed-2014-204067
May L, Gudger G, Armstrong P et al (2014) multisite exploration of clinical decision making for antibiotic use by emergency medicine providers using quantitative and qualitative methods. Infect Control Hosp Epidemiol. https://doi.org/10.1086/677637
Acknowledgements
We thank Alessio Vinicio Codella and Adelia Dora for their invaluable help in the study.
Funding
Open access funding provided by Università degli Studi della Campania Luigi Vanvitelli within the CRUI-CARE Agreement. No funding was received for this study.
Author information
Authors and Affiliations
Contributions
NC, LO, CM, and FGN were responsible for the conception and design of the study, interpreted the data, and wrote the paper; MM, LO, FC, and CM conducted the audits as Infectious Disease Consultants and contributed to collecting and analyzing the data; LO performed the statistical analysis; EA, VM, GB, AP, TR, GS participated in the audits and contributed to analyzing and interpreting the results; MdI collected the microbiological data, AC collected DDI data; MV supervised the program. All authors have read and agreed to the final version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
No conflict of interest declared.
Institutional review board statement
The study was approved by the Ethics Committee of the University of Campania L. Vanvitelli, Naples (n°17539i/2022).
Human and animal rights statement
All methods used in the study were in accordance with the international guidelines, with the standards on human experimentation of the Ethics Committee of the Azienda Ospedaliera Universitaria (AOU) L. Vanvitelli, and with the Helsinki Declaration of 1975, revised in 2013. The study was approved by Ethics Committee of the AOU L. Vanvitelli, University of Campania, Naples.
Informed consent
Informed consent was obtained from all participants included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Monari, C., Onorato, L., Allegorico, E. et al. The impact of a non-restrictive Antimicrobial Stewardship Program in the emergency department of a secondary-level Italian hospital. Intern Emerg Med 19, 493–500 (2024). https://doi.org/10.1007/s11739-023-03418-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11739-023-03418-1