Abstract
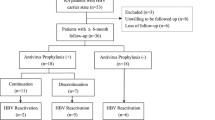
Conflicting results can be found in the literature on the frequency of hepatitis B virus (HBV) reactivation (HBVr) on rituximab (RTX) in rheumatic patients with previously resolved HBV (prHBV) infection. Here, we report the frequency of HBVr in a large historical cohort of caucasian rheumatic patients with prHBV receiving RTX. Registry data of rheumatic patients treated with RTX were retrospectively analysed. Demographic and clinical characteristics including evaluation of anti-HCV and HBV markers, annual HBV-DNA determination and aminotransferase levels assessed every three months, were recorded. Kaplan–Meier estimate was used to compare the risk of being still under therapy at different time points in patients with or without prHBV infection. Cox regression analysis was used to determine the association between recorded variables and treatment discontinuation. A total of 311 patients treated with RTX, 44 (14.1%) with and 267 (85.9%) without prHBV were analysed. No significant difference between the two groups regarding demographic and clinical characteristics was observed. During RTX treatment, detectable HBV-DNA and reappearance of HBsAg in patients with prHBV (seroreversion) were never observed. Kaplan–Meier functions were similar in patients with or without prHBV infection which was not associated with RTX discontinuation neither at univariate nor at multivariate analysis. These data are in favor of the concept that patients with rheumatologic diseases have a very low risk of reactivation of the HBV infection under RTX treatment. However, future prospective studies, including a larger number of patients, are still necessary to draw definitive conclusions.

Similar content being viewed by others
References
Leandro MJ, Cambridge G, Ehrenstein MR, Edwards JCW (2006) Reconstitution of peripheral blood B cells after depletion with rituximab in patients with rheumatoid arthritis. Arthritis Rheum 54(2):613–620. https://doi.org/10.1002/art.21617
Ferrari C, Missale G, Boni C, Urbani S (2003) Immunopathogenesis of hepatitis B. J Hepatol 39:36–42. https://doi.org/10.1016/S0168-8278(03)00137-5
Tan A, Bertoletti A (2015) Immune response in Hepatitis B virus infection. Cold Spring Harb Perspect Med 5:021428. https://doi.org/10.1101/cshperspect.a021428
Ferrari TC, Xavier MA, Vidigal PV, Amaral NS, Diniz PA, Resende AP, Miranda DM, Faria AC, Lima AS, Faria LC (2014) Occult hepatitis B virus infection in liver transplant patients in a Brazilian referral center. Braz J Med Biol Res 47(11):990–994
Hoofnagle JH (2009) Reactivation of hepatitis B. Hepatology 49(S5):S156–S165. https://doi.org/10.1002/hep.22945
Terrault NA, Lok ASF, McMahon BJ, Chang K-M, Hwang JP, Jonas MM, Brown RS Jr, Bzowej NH, Wong JB (2018) Update on prevention, diagnosis, and treatment of chronic hepatitis B: AASLD 2018 hepatitis B guidance. Hepatology 67(4):1560–1599. https://doi.org/10.1002/hep.29800
Guo L, Wang D, Ouyang X, Tang N, Chen X, Zhang Y, Zhu H, Li X (2018) Recent advances in HBV reactivation research. Biomed Res Int 2018:2931402. https://doi.org/10.1155/2018/2931402
Venerito V, Lopalco G, Cacciapaglia F, Fornaro M, Iannone F (2019) A Bayesian mixed treatment comparison of efficacy of biologics and small molecules in early rheumatoid arthritis. Clin Rheumatol 38(5):1309–1317. https://doi.org/10.1007/s10067-018-04406-z
Barone M, Notarnicola A, Lopalco G, Viggiani MT, Sebastiani F, Covelli M, Iannone F, Avolio AW, Di Leo A, Cantarini L, Lapadula G (2015) Safety of long-term biologic therapy in rheumatologic patients with a previously resolved hepatitis B viral infection. Hepatology 62(1):40–46. https://doi.org/10.1002/hep.27716
Venerito V, Angelini O, Fornaro M, Cacciapaglia F, Lopalco G, Iannone F (2021) A machine learning approach for predicting sustained remission in rheumatoid arthritis patients on biologic agents. JCR: J Clin Rheumatol Publish. https://doi.org/10.1097/rhu.0000000000001720
Evens AM, Jovanovic BD, Su YC, Raisch DW, Ganger D, Belknap SM, Dai MS, Chiu BC, Fintel B, Cheng Y, Chuang SS, Lee MY, Chen TY, Lin SF, Kuo CY (2011) Rituximab-associated hepatitis B virus (HBV) reactivation in lymphoproliferative diseases: meta-analysis and examination of FDA safety reports. Ann Oncol 22(5):1170–1180
Xu X, Shang Q, Chen X, Nie W, Zou Z, Huang A, Meng M, Jin L, Xu R, Zhang JY, Fu J, Wang L, Tang Z, Xie Y, Yang X, Zhang Z, Wang FS (2015) Reversal of B-cell hyperactivation and functional impairment is associated with HBsAg seroconversion in chronic hepatitis B patients. Cell Mol Immunol 12(3):309–316
Perrillo RP, Gish R, Falck-Ytter YT (2015) American Gastroenterological Association Institute technical review on prevention and treatment of hepatitis B virus reactivation during immunosuppressive drug therapy. Gastroenterology 148(1):221–244 e223. https://doi.org/10.1053/j.gastro.2014.10.038
Koutsianas C, Thomas K, Vassilopoulos D (2020) Reactivation of hepatitis B virus infection in rheumatic diseases: risk and management considerations. Ther Adv Musculoskelet Dis. 12:1759720X20912646. https://doi.org/10.1177/1759720X20912646
Mitroulis I, Hatzara C, Kandili A, Hadziyannis E, Vassilopoulos D (2013) Long-term safety of rituximab in patients with rheumatic diseases and chronic or resolved hepatitis B virus infection. Ann Rheum Dis 72(2):308–310. https://doi.org/10.1136/annrheumdis-2012-202088
Koutsianas C, Thomas K, Vassilopoulos D (2016) Prevention of HBV reactivation in patients treated with biologic agents. Expert Rev Clin Pharmacol 9(4):579–589. https://doi.org/10.1586/17512433.2016.1143773
van Vollenhoven R, Fleischmann R, Furst D, Lacey S, Lehane P (2015) Longterm safety of Rituximab: final report of the rheumatoid arthritis global clinical trial program over 11 years. J Rheumatol 42:150051. https://doi.org/10.3899/jrheum.150051
Varisco V, Viganò M, Batticciotto A, Lampertico P, Marchesoni A, Gibertini P, Pellerito R, Rovera G, Caporali R, Todoerti M, Covelli M, Notarnicola A, Atzeni F, Sarzi-Puttini P (2016) Low risk of hepatitis B virus reactivation in HBsAg-negative/Anti-HBc–positive carriers receiving rituximab for rheumatoid arthritis: a retrospective multicenter italian study. J Rheumatol 43(5):869–874. https://doi.org/10.3899/jrheum.151105
Tien YC, Yen HH, Chiu YM (2017) Incidence and clinical characteristics of hepatitis B virus reactivation in HBsAg-negative/HBcAb-positive patients receiving rituximab for rheumatoid arthritis. Clin Exp Rheumatol 35(5):831–836
Spinicci M, Emmi G, Dies L, Barilaro A, Vitiello G, Mencarini J, Cavallo A, Bartoloni A, Bartalesi F (2018) Short article: Safety of targeted prophylaxis strategy in patients with resolved hepatitis B virus infection receiving rituximab for immune-mediated diseases. Eur J Gastroenterol Hepatol 30(7):756–760. https://doi.org/10.1097/MEG.0000000000001132
Papalopoulos I, Fanouriakis A, Kougkas N, Flouri I, Sourvinos G, Bertsias G, Repa A, Avgoustidis N, Sidiropoulos P (2018) Liver safety of non-tumour necrosis factor inhibitors in rheumatic patients with past hepatitis B virus infection: an observational, controlled, long-term study. Clin Exp Rheumatol 36(1):102–109
Sugauchi F, Tanaka Y, Kusumoto S, Matsuura K, Sugiyama M, Kurbanov F, Ueda R, Mizokami M (2011) Virological and clinical characteristics on reactivation of occult hepatitis B in patients with hematological malignancy. J Med Virol 83:412–418. https://doi.org/10.1002/jmv.21995
Kuo MH, Tseng CW, Lee CH, Tung CH, Tseng KC, Lai NS (2020) Moderate risk of Hepatitis B virus reactivation in HBsAg-/HBcAb+ carriers receiving rituximab for rheumatoid arthritis. Sci Rep 10(1):2456
Samuel D, Forns X, Berenguer M, Trautwein C, Burroughs A, Rizzetto Trepo MC (2006) Report of the monothematic EASL conference on liver transplantation for viral hepatitis (Paris, France, January 12–14. J Hepatol 45(1):127–143. https://doi.org/10.1016/j.jhep.2006.05.001
Canzoni M, Marignani M, Sorgi ML, Begini P, Biondo MI, Caporuscio S, Colonna V, Casa FD, Conigliaro P, Marrese C, Celletti E, Modesto I, Peragallo MS, Lagana B, Picchianti-Diamanti A, Rosa RD, Ferlito C, Salemi S, D'Amelio R, Stroffolini T (2020) Prevalence of hepatitis B virus markers in patients with autoimmune inflammatory rheumatic diseases in Italy. Microorganisms 8(11). https://doi.org/10.3390/microorganisms8111792
Lopalco G, Venerito V, Cantarini L, Emmi G, Salaffi F, Di Carlo M, Tafuri S, Gentileschi S, Di Scala G, Nivuori M, Cacciapaglia F, Galeazzi M, Lapadula G, Iannone F (2019) Different drug survival of first line tumour necrosis factor inhibitors in radiographic and non-radiographic axial spondyloarthritis: a multicentre retrospective survey. Clin Exp Rheumatol 37(5):762–767
Venerito V, Natuzzi D, Bizzoca R, Lacarpia N, Cacciapaglia F, Lopalco G, Iannone F (2020) Serum sCD40L levels are increased in patients with psoriatic arthritis and are associated with clinical response to apremilast. Clin Exp Immunol 201(2):200–204. https://doi.org/10.1111/cei.13451
Funding
This research and its publication did not receive any specific funding.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
None of the authors have financial interests (employment, share ownership, patent rights, consultancy, research funding) in any company or institution that might benefit from this publication.
Data availability
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Barone, M., Venerito, V., Paolillo, R. et al. Long-term safety of rituximab in rheumatic patients with previously resolved hepatitis B virus infection. Intern Emerg Med 17, 475–480 (2022). https://doi.org/10.1007/s11739-021-02836-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11739-021-02836-3