Abstract
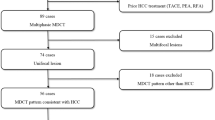
Ultrasound (US) detection of liver nodules in cirrhotic patients requires further radiological examinations and often a follow-up with repeated short-term evaluations to verify the presence of hepatocellular carcinoma (HCC). Aims of the study were to assess the rate of HCC diagnosis and to identify HCC predictors in a cohort of cirrhotics followed-up after US detection of the liver nodule(s). One-hundred-eighty-eight consecutive cirrhotic patients (124 males, mean age 64.2 years) with liver nodule(s) detected by US were enrolled. All patients underwent second-level imaging [computed tomography (TC) or magnetic resonance (MR)], and those without a definite diagnosis of HCC were followed-up with TC and/or RM repeated every 3–6 months up to 18 months if HCC was not diagnosed. After 18 months, non-HCC patients came back to routine US surveillance. HCC was diagnosed in 73/188 cases (38.8%). In 66/73 patients (90.4%) HCC was identified at first radiological evaluation after US, while in the remaining seven subjects it was diagnosed at the subsequent imaging examination. Age (p = 0.001) and nodule dimension (p = 0.0001) were independent predictors of HCC at multivariate analysis. Fourty-nine/188 patients were lost at follow up after 18 months. Twenty/139 remaining patients developed HCC and 3/139 cholangiocarcinoma; 77 died between 3 and 110 months from the beginning of the study (61 for end-stage liver disease, 8 for extrahepatic causes, eight for unknown causes). Patients who developed liver cancer earlier during the follow up had the shortest overall survival. US-detected liver nodules are not neoplastic in more than half of cirrhotic patients. A definite diagnosis may be obtained at the time of the first radiologic evaluation after US in the vast majority of the cases. Patients in whom nodules are found not to be tumoral may return to the US surveillance program routinely applied to all cirrhotics.



Similar content being viewed by others
References
Forner A, Llovet JM, Bruix J (2012) Hepatocellular carcinoma. Lancet 379:1245–1255. https://doi.org/10.1016/S0140-6736(11)61347-0
El-Serag HB (2011) Hepatocellular carcinoma. N Engl J Med 365:1118–1127. https://doi.org/10.1056/NEJMra1001683
Sherman M (2010) Hepatocellular carcinoma: epidemiology, surveillance, and diagnosis. Semin Liver Dis 30:3–16. https://doi.org/10.1055/s-0030-1247128
Schuppan D, Afdhal NH (2008) Liver cirrhosis. Lancet 37:838–851. https://doi.org/10.1016/S0140-6736(08)60383-9
Kokudo N, Takemura N, Hasegawa K et al (2019) Evidence-based clinical practice guidelines for Hepatocellular Carcinoma: the Japan Society of Hepatology 2013 update (3rd JSH-HCC Guidelines). Hepatol Res 49:1109–1113. https://doi.org/10.1111/hepr.13411
European Association for the Study of the Liver (2018) EASL clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol 69:182–236. https://doi.org/10.1016/j.jhep.2018.03.019
Marrero JA, Kulik LM, Sirlin CB et al (2018) Diagnosis, staging and management of hepatocellular carcinoma: 2018 practice guidance by the American Association for the Study of Liver Diseases. Hepatology 68:723–750. https://doi.org/10.1002/hep.29913
Zhang BH, Yang BH, Tang ZY (2004) Randomized controlled trial of screening for hepatocellular carcinoma. J Cancer Res Clin Oncol 130:417–422. https://doi.org/10.1007/s00432-004-0552-0
McMahon BJ, Bulkow L, Harpster A et al (2000) Screening for hepatocellular carcinoma in Alaska natives infected with chronic hepatitis B: a 16-year population-based study. Hepatology 32:842–846. https://doi.org/10.1053/jhep.2000.17914
Wong LL, Limm WM, Severino R, Wong LM (2000) Improved survival with screening for hepatocellular carcinoma. Liver Transpl 6:320–325. https://doi.org/10.1053/lv.2000.4875
Oka H, Kurioka N, Kim K et al (1990) Prospective study of early detection of hepatocellular carcinoma in patients with cirrhosis. Hepatology 12:680–687
Filomia R, Maimone S, Caccamo G et al (2016) Acute kidney injury in cirrhotic patients undergoing contrast-enhanced computed tomography. Medicine (Baltimore) 95:e4836. https://doi.org/10.1097/MD.0000000000004836
Rosado Ingelmo A, Doña Diaz I, Cabañas Moreno R et al (2016) Clinical practice guidelines for diagnosis and management of hypersensitivity reactions to contrast media. J Investig Allergol Clin Immunol 26:144–155. https://doi.org/10.18176/jiaci.0058
Guevara M, Fernandez-Esparrach G, Alessandria C et al (2004) Effects of contrast media on renal function in patients with cirrhosis: a prospective study. Hepatology 40:646–651. https://doi.org/10.1002/hep.20373
Nash K, Hafeez A, Hou S (2002) Hospital-acquired renal insufficiency. Am J Kidney Dis 39:930–936. https://doi.org/10.1053/ajkd.2002.32766
Najjar M, Hamad A, Salameh M, Agarwal A, Feinfeld DA (2002) The risk of radiocontrast nephropathy in patients with cirrhosis. Ren Fail 24:11–18
Morcos SK, Thomsen HS, Webb JA (1999) Contrast-media-induced nephrotoxicity: a consensus report. Contrast Media Safety Committee, European Society of Urogenital Radiology (ESUR). Eur Radiol 9:1602–1613
Brix G, Lechel U, Nekolla E, Griebel J, Becker C (2015) Radiation protection issues in dynamic contrast-enhanced (perfusion) computed tomography. Eur J Radiol 84:2347–2358. https://doi.org/10.1016/j.ejrad.2014.11.011
Rockey DC, Caldwell SH, Goodman ZD, Nelson RC, Smith AD; American Association for the Study of Liver Diseases (2009) Liver biopsy. Hepatology 49:1017–1044. https://doi.org/10.1002/hep.22742
Silva MA, Hegab B, Hyde C, Guo B, Buckels JA, Mirza DF (2008) Needle track seeding following biopsy of liver lesions in the diagnosis of hepatocellular cancer: a systematic review and meta-analysis. Gut 57:1592–1596. https://doi.org/10.1136/gut.2008.149062
Huang JF, Hsieh MY, Dai CY et al (2007) The incidence and risks of liver biopsy in non-cirrhotic patients: an evaluation of 3806 biopsies. Gut 56:736–737. https://doi.org/10.1136/gut.2006.115410
Cadranel JF, Rufat P, Degos F (2000) Practices of liver biopsy in France: results of a prospective nationwide survey. For the Group of Epidemiology of the French Association for the Study of the Liver (AFEF). Hepatology 32:477–481. https://doi.org/10.1053/jhep.2000.16602
Gilmore IT, Burroughs A, Murray-Lyon IM, Williams R, Jenkins D, Hopkins A (1995) Indications, methods, and outcomes of percutaneous liver biopsy in England and Wales: an audit by the British Society of Gastroenterology and the Royal College of Physicians of London. Gut 36:437–441
Sharma P, McDonald GB, Banaji M (1982) The risk of bleeding after percutaneous liver biopsy: relation to platelet count. J Clin Gastroenterol 4:451–453
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Statement of human and animal rights
This study was carried out following the international ethical recommendations for conducting research in humans in the latest revision of the Declaration of Helsinki. The study was approved by the Ethics Committee of the Messina University Hospital.
Informed consent
Written informed consent was obtained from each patient.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Franzè, M.S., Bottari, A., Caloggero, S. et al. Rate of hepatocellular carcinoma diagnosis in cirrhotic patients with ultrasound-detected liver nodules. Intern Emerg Med 16, 949–955 (2021). https://doi.org/10.1007/s11739-020-02541-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11739-020-02541-7