Abstract
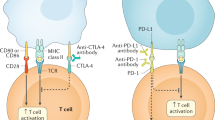
Immune checkpoint inhibitors (ICIs) have revolutionized cancer treatment. The number of indications is increasing and antibodies targeting the CTLA-4 and PD-1/PD-L1 pathways are now also prescribed in adjuvant settings and for metastatic cancer. However, ICIs reactivate autoreactive immune cells as well as tumour-specific T cells, which lead to immune-related adverse events (irAEs) in around 70% of treated patients. Although all organs can potentially be involved, the skin, gut, thyroid, lungs, liver, and joints are most frequently affected. Most irAEs occur in the first few months of treatment but late-onset toxicity—even after the ICI has been discontinued—is also possible. In terms of severity, most irAEs are grade 1–2. Some irAEs (especially myocarditis, pneumonitis, and encephalitis) are potentially fatal; in patients with highly suggestive clinical signs, treatment should be initiated before the diagnostic work-up has been completed. When confronted with an unexpected clinical sign, the physician must differentiate rapidly between an irAE, cancer progression, and another (unrelated) cause. The management of irAEs is based on the temporary or permanent discontinuation of the ICI and (for grade ≥ 2 events) the administration of steroids.
Similar content being viewed by others
References
Tivol EA et al (1995) Loss of CTLA-4 leads to massive lymphoproliferation and fatal multiorgan tissue destruction, revealing a critical negative regulatory role of CTLA-4. Immunity 3:541–547
Chen DS, Irving BA, Hodi FS (2012) Molecular pathways: next-generation immunotherapy—inhibiting programmed death-ligand 1 and programmed death-1. Clin Cancer Res 18:6580–6587
Weber J et al (2017) Adjuvant nivolumab versus Ipilimumab in resected Stage III or IV melanoma. N Engl J Med 377:1824–1835
Remon J, Besse B (2017) Immune checkpoint inhibitors in first-line therapy of advanced non-small cell lung cancer. Curr Opin Oncol 29:97–104
Wu Y et al (2017) The clinical value of combination of immune checkpoint inhibitors in cancer patients: a meta-analysis of efficacy and safety. Int J Cancer 141:2562–2570
Levy B et al (2019) Afatinib with pembrolizumab for treatment of patients with locally advanced/metastatic squamous cell carcinoma of the lung: the LUX-lung IO/KEYNOTE 497 study protocol. Clin Lung Cancer 20:e407–e412
Weber JS et al (2017) Safety profile of nivolumab monotherapy: a pooled analysis of patients with advanced melanoma. J Clin Oncol 35:785–792
Brahmer JR et al (2018) ASCO management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: american society of clinical oncology clinical practice guideline. J Clin Oncol 36:1714–1768
Haanen JBAG et al (2018) Management of toxicities from immunotherapy: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 29:iv264–iv266
Postow MA, Sidlow R, Hellmann MD (2018) Immune-related adverse events associated with immune checkpoint blockade. N Engl J Med 378:158–168
Gangadhar TC, Vonderheide RH (2014) Mitigating the toxic effects of anticancer immunotherapy. Nat Rev Clin Oncol 11:91–99
Naidoo J et al (2015) Toxicities of the anti-PD-1 and anti-PD-L1 immune checkpoint antibodies. Ann Oncol 26:2375–2391
Bowyer S et al (2016) Efficacy and toxicity of treatment with the anti-CTLA-4 antibody ipilimumab in patients with metastatic melanoma after prior anti-PD-1 therapy. Br J Cancer 114:1084–1089
Freeman GJ et al (2000) Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J Exp Med 192:1027–1034
Johnson DB et al (2016) Fulminant myocarditis with combination immune checkpoint blockade. N Engl J Med 375:1749–1755
Mahmood SS et al (2018) Myocarditis in patients treated with immune checkpoint inhibitors. J Am Coll Cardiol 71:1755–1764
Hu J-R et al (2019) Cardiovascular toxicities associated with immune checkpoint inhibitors. Cardiovasc Res 115:854–868
Wang DY et al (2018) Fatal toxic effects associated with immune checkpoint inhibitors: a systematic review and meta-analysis. JAMA Oncol 4:1721–1728
Salem J-E et al (2018) Cardiovascular toxicities associated with immune checkpoint inhibitors: an observational, retrospective, pharmacovigilance study. Lancet Oncol 19:1579–1589
Arangalage D et al (2017) Survival after fulminant myocarditis induced by immune-checkpoint inhibitors. Ann Intern Med 167:683–684
Touat M et al (2018) Immune checkpoint inhibitor-related myositis and myocarditis in patients with cancer. Neurology 91:e985–e994
Moslehi JJ, Salem J-E, Sosman JA, Lebrun-Vignes B, Johnson DB (2018) Increased reporting of fatal immune checkpoint inhibitor-associated myocarditis. Lancet 391:933
Zhang L et al (2019) Cardiotoxicity of immune checkpoint inhibitors. Curr Treat Options Cardiovasc Med 21:32
Gallegos C, Rottmann D, Nguyen VQ, Baldassarre LA (2019) Myocarditis with checkpoint inhibitor immunotherapy: case report of late gadolinium enhancement on cardiac magnetic resonance with pathology correlate. Eur Heart J Case Rep 3:yty149
Escudier M et al (2017) Clinical features, management, and outcomes of immune checkpoint inhibitor-related cardiotoxicity. Circulation 136:2085–2087
Lyon AR, Yousaf N, Battisti NML, Moslehi J, Larkin J (2018) Immune checkpoint inhibitors and cardiovascular toxicity. Lancet Oncol 19:e447–e458
Chung ES et al (2003) Randomized, double-blind, placebo-controlled, pilot trial of infliximab, a chimeric monoclonal antibody to tumor necrosis factor-alpha, in patients with moderate-to-severe heart failure: results of the anti-TNF Therapy Against Congestive Heart Failure (ATTACH) trial. Circulation 107:3133–3140
Daxini A, Cronin K, Sreih AG (2018) Vasculitis associated with immune checkpoint inhibitors-a systematic review. Clin Rheumatol 37:2579–2584
Kostine M et al (2019) Addressing immune-related adverse events of cancer immunotherapy: how prepared are rheumatologists? Ann Rheum Dis 78:860–862
Le Burel S et al (2017) Prevalence of immune-related systemic adverse events in patients treated with anti-programmed cell death 1/anti-programmed cell death-ligand 1 agents: a single-centre pharmacovigilance database analysis. Eur J Cancer 82:34–44
Spain L, Diem S, Larkin J (2016) Management of toxicities of immune checkpoint inhibitors. Cancer Treat Rev 44:51–60
Cappelli LC, Gutierrez AK, Bingham CO, Shah AA (2017) Rheumatic and musculoskeletal immune-related adverse events due to immune checkpoint inhibitors: a systematic review of the literature. Arthritis Care Res (Hoboken) 69:1751–1763
Ramos-Casals M et al (2019) Sicca/Sjögren’s syndrome triggered by PD-1/PD-L1 checkpoint inhibitors. Data from the international immunocancer registry (ICIR). Clin Exp Rheumatol 37(Suppl 118):114–122
Anastasopoulou A, Ziogas DC, Samarkos M, Kirkwood JM, Gogas H (2019) Reactivation of tuberculosis in cancer patients following administration of immune checkpoint inhibitors: current evidence and clinical practice recommendations. J Immunother Cancer 7:239
Bernard-Tessier A et al (2017) Immune-related eosinophilia induced by anti-programmed death 1 or death-ligand 1 antibodies. Eur J Cancer (Oxford, England 1990) 81:135–137
Michot JM et al (2018) Cytopénies immunologiques induites par des anticorps anti-PD-1 ou anti-PD-L1: une étude observationnelle descriptive. La Revue de Médecine Interne 39:A35–A36
Delanoy N et al (2019) Haematological immune-related adverse events induced by anti-PD-1 or anti-PD-L1 immunotherapy: a descriptive observational study. Lancet Haematol 6:e48–e57
Davis EJ et al (2019) Hematologic complications of immune checkpoint inhibitors. Oncologist 24:584–588
Michot JM et al (2019) Haematological immune-related adverse events with immune checkpoint inhibitors, how to manage? Eur J Cancer 122:72–90
Okawa S et al (2019) Pembrolizumab-induced autoimmune hemolytic anemia and hemophagocytic lymphohistiocytosis in non-small cell lung cancer. Intern Med 58:699–702
Dutertre M, de Menthon M, Noël N, Albiges L, Lambotte O (2019) Cold agglutinin disease as a new immune-related adverse event associated with anti-PD-L1s and its treatment with rituximab. Eur J Cancer 110:21–23
Shiuan E et al (2017) Thrombocytopenia in patients with melanoma receiving immune checkpoint inhibitor therapy. J Immunother Cancer 5:8
Neunert C et al (2019) American society of hematology 2019 guidelines for immune thrombocytopenia. Blood Adv 3:3829–3866
Cuzzubbo S et al (2017) Neurological adverse events associated with immune checkpoint inhibitors: review of the literature. Eur J Cancer 73:1–8
Larkin J et al (2017) Neurologic serious adverse events associated with Nivolumab plus ipilimumab or Nivolumab alone in advanced melanoma, including a case series of encephalitis. Oncologist 22:709–718
Matsuoka H et al (2018) Nivolumab-induced limbic encephalitis with anti-Hu antibody in a patient with advanced pleomorphic carcinoma of the lung. Clin Lung Cancer 19:e597–e599
Brown MP, Hissaria P, Hsieh AH, Kneebone C, Vallat W (2017) Autoimmune limbic encephalitis with anti-contactin-associated protein-like 2 antibody secondary to pembrolizumab therapy. J Neuroimmunol 305:16–18
Kolb NA et al (2018) Neuromuscular complications of immune checkpoint inhibitor therapy. Muscle Nerve. https://doi.org/10.1002/mus.26070
Chen Q et al (2018) Fatal myocarditis and rhabdomyolysis induced by nivolumab during the treatment of type B3 thymoma. Clin Toxicol (Phila) 56:667–671
Parlati L et al (2018) Incidence of grade 3–4 liver injury under immune checkpoints inhibitors: a retrospective study. J Hepatol 69:1396–1397
De Martin E et al (2018) Characterization of liver injury induced by cancer immunotherapy using immune checkpoint inhibitors. J Hepatol 68:1181–1190
Huffman BM, Kottschade LA, Kamath PS, Markovic SN (2018) Hepatotoxicity after immune checkpoint inhibitor therapy in melanoma: natural progression and management. Am J Clin Oncol 41:760–765
Soularue E et al (2018) Enterocolitis due to immune checkpoint inhibitors: a systematic review. Gut 67:2056–2067
Johnson DB et al (2016) Ipilimumab therapy in patients with advanced melanoma and preexisting autoimmune disorders. JAMA Oncol 2:234–240
Duma N et al (2019) Sex differences in tolerability to anti-programmed cell death protein 1 therapy in patients with metastatic melanoma and non-small cell lung cancer: are we all equal? Oncologist. https://doi.org/10.1634/theoncologist.2019-0094
Sukari A et al (2019) Cancer site and adverse events induced by immune checkpoint inhibitors: a retrospective analysis of real-life experience at a single institution. Anticancer Res 39:781–790
Asher N et al (2019) Recurrent pneumonitis in patients with melanoma treated with immune checkpoint inhibitors. Oncologist 24:640–647
Barroso-Sousa R et al (2018) Incidence of endocrine dysfunction following the use of different immune checkpoint inhibitor regimens: a systematic review and meta-analysis. JAMA Oncol 4:173–182
Marlier J, Cocquyt V, Brochez L, Van Belle S, Kruse V (2014) Ipilimumab, not just another anti-cancer therapy: hypophysitis as side effect illustrated by four case-reports. Endocrine 47:878–883
Kapke J, Shaheen Z, Kilari D, Knudson P, Wong S (2017) Immune checkpoint inhibitor-associated type 1 diabetes mellitus: case series, review of the literature, and optimal management. Case Rep Oncol 10:897–909
de Filette J, Andreescu CE, Cools F, Bravenboer B, Velkeniers B (2019) A systematic review and meta-analysis of endocrine-related adverse events associated with immune checkpoint inhibitors. Horm Metab Res 51:145–156
Sibaud V (2018) Dermatologic reactions to immune checkpoint inhibitors : skin toxicities and immunotherapy. Am J Clin Dermatol 19:345–361
Dai J, Belum VR, Wu S, Sibaud V, Lacouture ME (2017) Pigmentary changes in patients treated with targeted anticancer agents: a systematic review and meta-analysis. J Am Acad Dermatol 77:902–910.e2
Belum VR et al (2016) Characterisation and management of dermatologic adverse events to agents targeting the PD-1 receptor. Eur J Cancer 60:12–25
Hua C et al (2016) Association of vitiligo with tumor response in patients with metastatic melanoma treated with pembrolizumab. JAMA Dermatol 152:45–51
Nayar N, Briscoe K, Fernandez Penas P (2016) Toxic epidermal necrolysis-like reaction with severe satellite cell necrosis associated with Nivolumab in a patient with Ipilimumab refractory metastatic melanoma. J Immunother 39:149–152
Abdel-Rahman O et al (2017) Immune-related ocular toxicities in solid tumor patients treated with immune checkpoint inhibitors: a systematic review. Expert Rev Anticancer Ther 17:387–394
Bitton K et al (2019) Prevalence and clinical patterns of ocular complications associated with anti-PD-1/PD-L1 anticancer immunotherapy. Am J Ophthalmol 109–117
Cortazar FB et al (2016) Clinicopathological features of acute kidney injury associated with immune checkpoint inhibitors. Kidney Int 90:638–647
Tang PA, Heng DYC (2013) Programmed death 1 pathway inhibition in metastatic renal cell cancer and prostate cancer. Curr Oncol Rep 15:98–104
Fadel F, El Karoui K, Knebelmann B (2009) Anti-CTLA4 antibody-induced lupus nephritis. N Engl J Med 361:211–212
Tison A et al (2019) Safety and efficacy of immune checkpoint inhibitors in patients with cancer and preexisting autoimmune disease: a nationwide multicenter cohort study. Arthritis Rheumatol. https://doi.org/10.1002/art.41068
Gutzmer R et al (2017) Programmed cell death protein-1 (PD-1) inhibitor therapy in patients with advanced melanoma and preexisting autoimmunity or ipilimumab-triggered autoimmunity. Eur J Cancer 75:24–32
Okazaki T, Honjo T (2007) PD-1 and PD-1 ligands: from discovery to clinical application. Int Immunol 19:813–824
Ravi S et al (2014) Ipilimumab administration for advanced melanoma in patients with pre-existing Hepatitis B or C infection: a multicenter, retrospective case series. J Immunother Cancer 2:33
Sharma A, Thompson JA, Repaka A, Mehnert JM (2013) Ipilimumab administration in patients with advanced melanoma and hepatitis B and C. J Clin Oncol 31:e370–372
Uldrick TS et al (2019) Assessment of the safety of pembrolizumab in patients with HIV and advanced cancer-a phase 1 study. JAMA Oncol. https://doi.org/10.1001/jamaoncol.2019.2244
Simonaggio A et al (2019) Evaluation of readministration of immune checkpoint inhibitors after immune-related adverse events in patients with cancer. JAMA Oncol. https://doi.org/10.1001/jamaoncol.2019.1022
Michot J-M et al (2020) The ImmunoTOX multidisciplinary board: a descriptive study of collaborative management of immune-related adverse events. Eur J Cancer
Champiat S et al (2016) Management of immune checkpoint blockade dysimmune toxicities: a collaborative position paper. Ann Oncol 27:559–574
Peyrony O et al (2019) Immune checkpoint blockade toxicity among patients with cancer presenting to the emergency department. Emerg Med J 36:306–309
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Prof Olivier Lambotte: paid expert testimony and consultancy fees from BMS France, MSD, and Astra Zeneca; consultancy fees from Incyte, expert testimony for Janssen, all outside the submitted work. Dr Noel has received speaker fees from MSD outside the submitted work.
Statement of human and animal rights
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed consent
For this type of study, a review, formal consent is not required.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
de La Rochefoucauld, J., Noël, N. & Lambotte, O. Management of immune-related adverse events associated with immune checkpoint inhibitors in cancer patients: a patient-centred approach. Intern Emerg Med 15, 587–598 (2020). https://doi.org/10.1007/s11739-020-02295-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11739-020-02295-2