Abstract
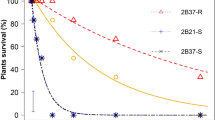
Quantifying the level of seed germiabiliy of herbicide-resistant (R) and susceptible (S) weeds is useful for understanding the evolutionary development of herbicide resistance, but also for implementing herbicide-resistance management strategies. Germination is a crucial aspect in the life phase of weeds. Phalaris brachystachys biotypes resistant to ACCase-inhibiting herbicides have been confirmed in wheat fields in Iran. This study aimed to investigate the germination behaviour of ACCase- resistant and susceptible subpopulations P. brachystachys under different environmental factors. An analysis of the seed germination of P. brachystachys sub populations at the seed stage was therefore conducted. The resistant (R) and susceptible (S) P. brachystachys subpopulation germination traits were tested in different temperature, salinity stress, drought, and burial depth conditions. All tests were carried out with five replications in a completely randomized design. The highest germination percentage in both subpopulations occurred at a temperature range of 20 to 25 °C. At 35 °C, no germination occurred. In terms of estimated cardinal temperatures, no differences were observed between S and R subpopulations. Seeds containing the ACCase Ile-1781-Thr mutation germinated better under salt and osmotic stress. There were no fitness differences in pH conditions. The percentage and rate of emergence of the R subpopulation were more than S subpopulation at different burial depths. The seedling emergence reached a maximum of 50% in the S subpopulation at a depth of 6.65 cm and in the R subpopulation at depths of 9.81 cm, respectively. Significant differences in seed germination were found between herbicide-resistant and susceptible sub population, and the pleiotropic effect of resistant alleles on germination and seed emergence under different environmental conditions was demonstrated. Results suggested that deep tillage operations and the delayed sowing of autumn-sown crops could control resistant populations that emerge more rapidly than S population; therefore, the prevalence in the R population may decrease.





Similar content being viewed by others
Data availability
All data generated or analyzed during this study are included in this published article. All data related to the present work can be obtained through Email: gherekhloo@gau.ac.ir.
References
Alcantra C, Jimenez-Hidalgo MJ, Saavedra M (2020) Responses of Phalaris minor Rezt and Phalaris brachystachys Link to different levels of soil water availability. Span J Agric Res 8(4):1074–1083
Alimagham SM, Ghaderi-Far F (2014) Hydrotime model: introduction and application of this model in seed researches. Env Stresses Crop Sci 7:41–52
Argyris J, Dahal P, Hayashi E, Still DW, Bradford KJ (2008) Genetic variation for lettuce seed thermoinhibition is associated with temperature sensitive expression of abscisic acid, gibberellin, and ethylene biosynthesis, metabolism, and response genes. Plant Physiol 148(2):926–947
Bradford KJ, Still DW (2004) Applications of hydrotime analysis in seed testing. Seed Technol 26:75–85
CABI (2021) Invasive Species Compendium. Wallingford, UK: CAB International. www.cabi.org/isc
Chachalis D, Korres N, Khah EM (2008) Factors affecting seed germination and emergence of Venice mallow (Hibiscus trionum). Weed Sci 56:509–515
Chhokar RS, Sharma RK (2008) Multiple herbicide resistance in little seed canary grass (Phalaris minor): a threat to wheat production in India. Weed Biol Manag 8:112–123
Cousens RD, Fournier-Level A (2018) Herbicide resistance costs: what are we actually measuring and why? Pest Manag Sci 74(7):1539–1546
Délye C, Menchari Y, Michel S, Cadet E, Le Corre V (2013) A new insight into arable weed adaptive evolution: mutations endowing herbicide resistance also affect germination dynamics and seedling emergence. Ann Bot 111(4):681–691
Du L, Bai S, Li Q, Qu M, Yuan G, Guo W (2017) Effect of herbicide resistance endowing three ACCase mutations on seed germination and viability in American slough grass (Beckmannia syzigachne Steud Fernald). Chil J Agric Res 77(2):142–149
Forcella F, Benech-Arnold RL, Sanchez R, Ghersa CM (2000) Modelling seedling emergence. Field Crops Res 67:123–139
Ghaderi-Far F, Gherekhloo J, Alimagham SM (2010) Influence of environmental factors on seed germination and seedling emergence of yellow sweet clover (Melilotus officinalis). Planta Daninha 28(3):436–469
Golmohammadzadeh S, Gherekhloo J, Rojano-Delgado AM, Osuna-Ruiz MD, Kamkar B, Ghaderi-Far F, De Prado R (2019) The first case of short-spiked canary grass (Phalaris brachystachys) with cross-resistance to ACCase-inhibiting herbicides in Iran. Agron 9:377–389
Golmohammadzadeh S, Rojano-Delgado AM, Vázquez-García JG, Romano Y, Osuna MD, Gherekhloo J, De Prado R (2020) Cross-resistance mechanisms to ACCase-inhibiting herbicides in short-spike canarygrass (Phalaris brachystachys). Plant Physiol Biochem 15:681–688
Golmohammadzadeh S, Gherekhloo J, Osuna-Ruiz MD, Ghaderi-Far F, Kamkar B, Alcántara-de la Cruz R, De Prado R (2022) Physiological fitness associated to ACCase target-site resistance enhances growth and reproduction in Phalaris brachystachys. Agron 12:1206
Gonzalez-Andujar JL, Jimenez-Hidalgo M, Garcia-Torres L, Saavedra M (2005) Demography and population dynamic of the arable weed Phalaris brachystachys L. (short-spiked canary grass) in winter wheat. Crop Prot 24(6):581–584
Hassanpour-bourkheili S, Gherekhloo J, Kamkar B, Ramezanpour SS (2021) No fitness cost associated with Asn-2041-Ile mutation in winter wild oat (Avena ludoviciana) seed germination under various environmental conditions. Sci Rep 11:1572
Hawkins KK, Allen P, Meyer S (2013) Secondary dormancy of seeds in relation to the Bromus tectorum-Pyrenophorasemeniperda pathosystem. Plant Prot Sci 49:11–14
Hu XW, Wang YR, Wu YP (2008) Effects of the pericarp on imbibition, seed germination, and seedling establishment in seeds of Hedysarum scoparium Fisch. et Mey. Ecol Res 24:559–564
ISTA (1985) International rules for seed testing. Seed Sci Technol 13:307–520
Javaid MM, Tanveer A (2014) Germination ecology of Emex spinosa and Emex australis, invasive weeds of winter crops. Weed Res 54:565–575
Keshtkar E, Mathiassen SK, Beffa R, Kudsk P (2017) Seed Germination and seedling emergence of blackgrass (Alopecurus myosuroides) as affected by non–target-site herbicide resistance. Weed Sci 65:732–742
Keshtkar E, Abdolshahi R, Sasanfar H, Zand E, Beffa R, Kudsk DFE (2018) Assessing fitness costs from a herbicide resistance management perspective: a review and insight. Weed Sci 67(2):137–148
Lee YP, Baek KH, Lee HS, Kwak SS, Bang JW, Kwon SY (2010) Tobacco seeds simultaneously overexpressing Cu/Zn-superoxide dismutase and ascorbate peroxidase display enhanced seed longevity and germination rates under stress conditions. J Exp Bot 61:2499–2506
Li Q, Tan JN, Li W, Yuan GH, Du L, Ma S, Wang JX (2015) Effects of environmental factors on seed germination and emergence of Japanese brome (Bromus japonicus). Weed Sci 63:1–10
Liu W, Bai S, Zhao N, Li W, Zhang L, Wang J (2018) Non-target site-based resistance to tribenuron-methyl and essential involved genes in Myosotona quaticum (L.). BMC Plant Biol 18:225–237
Matzrafi M, Gerson O, Rubin B, Peleg Z (2017) Different mutations endowing resistance to Acetyl-CoA Carboxylase Inhibitors results in changes in ecological fitness of Lolium rigidum populations. Front Plant Sci 8:1078
Menchari Y, Chauvel B, Darmency H, Delye C (2008) Fitness cost associated with three mutant acetyl-coenzyme A carboxylase alleles endowing herbicide resistance in blackgrass Alopecurus myosuroides. J Appl Ecol 45:939–947
Micheal BE, Kaufman MR (1973) The osmotic potential of polyethylene glycol 6000. Plant Physiol 51:914–916
Oram RN (2004) Phalaris canariensis is a domesticated form of P. brachystachys. Genet Resour Crop Evol 51(3):259–267
Owen MJ, Michael PJ, Renton M, Steadman KJ, Powles SB (2011) Towards large-scale prediction of Lolium rigidum emergence. II. Correlation between dormancy and herbicide resistance levels suggests an impact of cropping systems. Weed Res 51:133–141
Panozzo S, Scarabel L, Rosan V, Sattin M (2017) A new Ala-122-Asn amino acid change confers decreased fitness to ALS-resistant Echinochloa crus-galli. Front Plant Sci 8:2042
Recasens J, Royo-Esnal A, Valencia-Gredilla F, Torra J (2020) Efficiency, profitability and carbon footprint of different management programs under no-till to control herbicide resistant Papaver rhoeas. Plants 9:433
Sheng GS, Vila-Aiub M, Busi R, Goggin D, Powles S (2019) Physiological Fitness Cost Associated with Glyphosate Resistance in Echinochloa colona: Seed Germination Ecology. J Trop Plant Physiol 11:1–12
Shrestha A, Desouza LL, Yang P, Sosnoskie L, Hanson BD (2018) Differential tolerance of glyphosate-susceptible and glyphosate-resistant biotypes of jungle rice (Echinochloa colona) to environments during germination, growth, and intraspecific competition. Weed Sci 66:340–346
Siani RK, Malone J, Preston C, Gill G (2017) Persistence of resistance alleles 1781, 2041, and 2078 in the absence of herbicide selection. Agron J 109:1–5
Soltani A, Robertson MJ, Torabi B, Yousefi-Daz M, Sarparast R (2006) Modelling seedling emergence in chickpea as influenced by temperature and sowing depth. Agric Forest Meteorol 138:156–167
Tang W, Zhou F, Chen J, Zhou X (2019) Resistance to ACCase-inhibiting herbicides in an Asia minor bluegrass (Polypogon fugax) population in China. Pestic Biochem Physiol 108:16–20
Torres-Garcia JR, Uscanga-Mortera E, Trejo C, Conde-Martinez V, Kohashi-Shibata J, Nunes-Farfan J, Martinez-Moreno D (2015) Effect of herbicide resistance on seed physiology of Phalaris minor (littleseed canarygrass). Botan Sci 93(3):661–667
Travlos I, Gazoulis I, Kanatas P, Tsekoura A, Zannopoulos S, Papastylianou P (2020) Key factors affecting weed seeds’ germination, weed emergence, and their possible role for the efficacy of false seedbed technique as weed management practice. Front Agron 2:1–9
Uddin MN, Hossain MA, Burritt DJ (2016) Salinity and drought stress: similarities and differences in oxidative responses and cellular redox regulation. In: Ahmad P (ed) Water stress and crop plants a sustainable approach. John Wiley & Sons, Singapore, pp 86–101
Verloove F (2006) Catalogue of neophytes in Belgium (1800–2005). National Botanic Garden of Belgium, Meise, Belgium, pp 89–94
Vila-Aiub MM, Neve P, Steadman KJ, Powles SB (2005) Ecological fitness of a multiple herbicide-resistant Lolium rigidum population: dynamics of seed germination and seedling emergence of resistant and susceptible phenotypes. J Appl Ecol 42:288–298
Vila-Aiub MM, Neve P, Powles SB (2009) Fitness costs associated with evolved herbicide resistance genes in plants. New Phytol 184:751–767
Vila-Aiub MM, Neve P, Roux F (2011) A unified approach to the estimation and interpretation of resistance costs in plants. Heredity 107(5):386–394
Vila-Aiub MM, Gundel PE, Preston C (2015) Experimental methods for estimation of plant fitness costs associated with herbicide-resistance genes. Weed Sci 63:203–216
Wang T, Picard JC, Tian X, Darmency H (2020) A herbicide-resistant ACCase 1781 Setaria mutant shows higher fitness than wild type. Herediy 105:394–400
Wu X, Zhang T, Pan L, Wang L, Xu H, Dong L (2016) Germination requirements differ between fenoxaprop-P-ethyl resistant and susceptible Japanese foxtail (Alopecurus japonicus) biotypes. Weed Sci 64(4):653–663
Zhao N, Li Q, Guo W, Zhang L, Ge L, Wang J (2017) Effect of Environmental factors on germination and emergence of Shortawn Foxtail (Alopecurus aequalis). Weed Sci 66(1):47–56
Acknowledgements
The authors wish to thank the University of Cordoba, Spain and the Gorgan University of Agricultural Science and Natural Resource (GUANR).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no potential conflict of interest.
Additional information
Communicated by F. Araniti.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Golmohammadzadeh, S., Gherekhloo, J., Ghaderi-Far, F. et al. Germination biology of susceptible and target-site (Ile-1781-Thr) herbicide resistant short-spiked canary grass (Phalaris brachystachys) subpopulations. Acta Physiol Plant 46, 9 (2024). https://doi.org/10.1007/s11738-023-03640-6
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11738-023-03640-6