Abstract
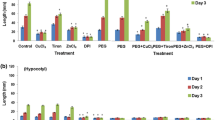
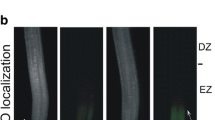
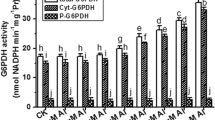
Besides gravity, roots are also guided by light to grow deep into the soil and sensitivity of roots to light is evidently due to presence of photoreceptors like phototropins. Such light-induced root growth (light-escape growth) presumably involves reactive oxygen species (ROS). Present study explores the possibility of ROS action in this event during early seedling growth of Vigna radiata based on pharmacological evidences. Germinated (20 h) seeds were incubated in dark or light in presence of general ROS scavenger (propyl gallate), specific scavengers of \({\text{O}}_{2}^{ \cdot \; - }\) (copper chloride; CuCl2), H2O2 [dimethylthiourea (DMTU) and potassium iodide (KI)] and ˙OH (sodium benzoate) and ROS-producing enzyme inhibitors [zinc chloride (ZnCl2), inhibitor of NADPH oxidase (NOX); diethyldithiocarbamate (DEDTC), inhibitor of superoxide dismutase (SOD) and salicylhydroxamic acid (SHAM), inhibitor of peroxidase]. Light-induced root growth of 3-day seedlings diminished significantly in case of all the treatments suggesting for a positive role of ROS in light-escape growth. This is supported by elevated level of apoplastic ROS in light grown roots as evident from ROS-specific staining [nitroblue tetrazolium chloride (NBT) for \({\text{O}}_{2}^{ \cdot \; - }\) and 3,3,5,5-tetramethylbenzidine (TMB) for H2O2] and spectrophotometric estimation of apoplastic ROS production (\({\text{O}}_{2}^{ \cdot \; - }\) and H2O2). In addition, higher activity of membrane bound NOX (producing \({\text{O}}_{2}^{ \cdot \; - }\)) and apoplastic class III peroxidase (Prx, producing ˙OH) in light grown roots further corroborates the view that apoplastic ROS (initiated with NOX-generated \({\text{O}}_{2}^{ \cdot \; - }\), which is converted, either spontaneously or by the activity of SOD, to H2O2 and further metabolized by Prx to ˙OH that participates in cell wall relaxation required for growth) is instrumental in light-escape growth of roots.






Similar content being viewed by others
References
Airianah OB, Vreeburg RAM, Fry SC (2016) Pectic polysaccharides are attacked by hydroxyl radicals in ripening fruit: evidence from a fluorescent fingerprinting method. Ann Bot 117:441–455. https://doi.org/10.1093/aob/mcv192
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Burbach C, Markus K, Zhang Y, Schlicht M, Baluška F (2012) Photophobic behavior of maize roots. Plant Signal Behav 7:874–878
Carter C, Healy R, O’Tool NM, Saqlan Naqvi SM, Ren G, Park S, Beattie GA, Horner HT, Thornburg RW (2007) Tobacco nectarines express a novel NADPH oxidase implicated in the defense of floral reproductive tissues against microorganisms. Plant Physiol 143:389–399. https://doi.org/10.1104/pp.106.089326
Causin HF, Roqueiro G, Petrillo E, Lainez V, Pena LB, Marchetti CF, Gallego SM, Maldonado SI (2012) The control of root growth by reactive oxygen species in Salix nigra Marsh. seedlings. Plant Sci 183:197–205. https://doi.org/10.1016/j.plantsci.2011.08.012
Černý M, Habánová H, Berka M, Luklová M, Brzobohatý B (2018) Hydrogen peroxide: its role in plant biology and crosstalk with signalling networks. Int J Mol Sci 19(2812):1–30. https://doi.org/10.3390/ijms19092812
Das S, Kar RK (2017) Reactive oxygen species-mediated promotion of root growth under mild water stress during early seedling stage of Vigna radiata (L.) Wilczek. J Plant Growth Regul 36:338–347
Fick GN, Qualset CO (1975) Genetic control of endosperm amylase activity and gibberellic acid responses in standard-height and short-statured wheats. Proc Natl Acad Sci USA 72:892–895
Foyer C (2018) Reactive oxygen species, oxidative signaling and the regulation of photosynthesis. Environ Exp Bot 154:134–142. https://doi.org/10.1016/j.envexpbot.2018.05.003
Frahry G, Schopfer P (2001) NADH-stimulated, cyanide-resistant superoxide production in maize coleoptiles analyzed with a tetrazolium-based assay. Planta 212:175–183
Galen C, Rabenold JJ, Liscum E (2007a) Light-sensing in roots. Plant Signal Behav 2:106–108
Galen C, Rabenold JJ, Liscum E (2007b) Functional ecology of a blue light photoreceptor: effects of phototropin-1 on root growth enhance drought tolerance in Arabidopsis thaliana. New Phytol 173:91–99
Garg N, Manchanda G (2009) ROS generation in plants: boon or bane? Plant Biosyst 143:81–96
Gay C, Gebicki JM (2000) A critical evaluation of the effect of sorbitol on the ferric-xylenol orange hydroperoxide assay. Anal Biochem 284:217–220
Gelderen KV, Kang C, Pierik R (2018) Light signaling, root development, and plasticity. Plant Physiol 176:1049–1060
Georgiou C, Tairis N, Sotiropoulou A (2000) Hydroxyl radical scavengers inhibit lateral-type sclerotial differentiation and growth in phytopathogenic fungi. Mycologia 92(5):825–834. https://doi.org/10.2307/3761577
Hejl AM, Koster KL (2004) Juglone disrupts root plasma membrane H+-ATPase activity and impairs water uptake, root respiration and growth in soybean (Glycine max) and corn (Zea mays). J Chem Ecol 30:453–471
Kar RK (2011) Plant responses to water stress, role of reactive oxygen species. Plant Signal Behav 6:1741–1745
Liszkay A, van der Zalm E, Schopfer P (2004) Production of reactive oxygen intermediates (O2⋅−, H2O2, and OH⋅) by maize roots and their role in wall loosening and elongation growth. Plant Physiol 136:3114–3123
Lushchak V, Semchyshyn H, Lushchak O, Mandryk S (2006) Diethyldithiocarbamate inhibits in vivo Cu, Zn-superoxide dismutase and perturbs free radical processes in the yeast Saccharomyces cerevisiae cells. Biochem Biophys Res Comm 338:1739–1744. https://doi.org/10.1016/j.bbrc.2005.10.147
Majumdar A, Kar RK (2016) Integrated role of ROS and Ca2+ in blue light-induced chloroplast avoidance movement in leaves of Hydrilla verticillata (L.f) Royle. Protoplasma 253:1529–1539. https://doi.org/10.1007/s00709-015-0911-5
Majumdar A, Kar RK (2018) Congruence between PM H+-ATPase and NADPH oxidase during root growth: a necessary probability. Protoplasma 255:1129–1137. https://doi.org/10.1007/s00709-018-1217-1
Majumdar A, Kar RK (2019) Orchestration of Cu-Zn SOD and class III peroxidase with upstream interplay between NADPH oxidase and PM H+-ATPase mediates root growth in Vigna radiata (L.) Wilczek. J Plant Physiol 232:248–256. https://doi.org/10.1016/j.jplph.2018.11.001
Majumdar A, Kar RK (2020) Chloroplast avoidance movement: a novel paradigm of ROS signalling. Photosynth Res 144:109–121. https://doi.org/10.1007/s11120-020-00736-9
Mandoli DF, Briggs WR (1982) Optical properties of etiolated plant tissues. Proc Natl Acad Sci USA 79:2902–2906
Minibayeva F, Kolesnikov O, Chasov A, Beckett R, Luthje S, Vylegzhanina N, Buck F, Bottger M (2009) Wound-induced apoplastic peroxidase activities: their roles in the production and detoxification of reactive oxygen species. Plant Cell Environ 32(5):497–508
Misra PA, Fridovich I (1972) The role of superoxide anion in the autoxidation of epinephrine and a simple assay for superoxide dismutase. J Biol Chem 247:3170–3175
Mittler R (2017) ROS are good. Trends Plant Sci 22:11–19. https://doi.org/10.1016/j.tplants.2016.08.002
Mo M, Yokawa K, Wan Y, Baluska F (2015) How and why do root apices sense light under the soil surface? Frontiers Plant Sci. https://doi.org/10.3389/fpls.2015.00775
Moothoo-Padayachie A, Varghese B, Pammenter NW, GovenderSershen P (2016) Germination associated ROS production and glutathione redox capacity in two recalcitrant-seeded species differing in seed longevity. Botany 94(12):1103–1114. https://doi.org/10.1139/cjb-2016-0130
Neel JK (1948) A limnological investigation of the Psammon in Douglas Lake, Michigan, with especial reference to shoal and shore line dynamics. Trans Am Micr Soc 67:1–53
Paddock T, Lima D, Mason ME, Apel K, Armstrong GA (2012) Arabidopsis light-dependent protochlorophyllide oxidoreductase A (PORA) is essential for normal plant growth and development. Plant Mol Biol 78:447–460
Padmanabhan MS, Dinesh-Kumar SP (2010) All hands on deck—the role of chloroplasts, endoplasmic reticulum, and the nucleus in driving plant innate immunity. Mol Plant Microbe Interact 23(11):1368–1380. https://doi.org/10.1094/MPMI-05-10-0113
Perkins EJ (1963) Penetration of light into littoral soils. J Ecol 51:687–692
Podgorska A, Burian M, Szal B (2017) Extra-cellular but extra-ordinarily important for cell: apoplastic reactive oxygen species metabolism. Front Plant Sci 8:1353
Qin L, Walk TC, Han P, Chen L, Zhang S, Li Y, Hu X, Xie L, Yang Y, Liu J, Lu X, Yu C, Tian J, Shaff JE, Kochian LV, Liao X, Liao H (2019) Adaptation of roots to nitrogen deficiency revealed by 3D quantification and proteomic analysis. Plant Physiol 179:329–347
Rodrigues ML, Pacheco CA, Chaves MM (1995) Soil-plant relations, root distribution and biomass partitioning in Lupinus albus L. under drought conditions. J Exp Bot 46:947–956
Sagi M, Fluhr R (2001) Superoxide production by plant homologues of the gp91(phox) NADPH oxidase. Modulation of activity by calcium and by tobacco mosaic virus infection. Plant Physiol 126:1281–1290
Schopfer P (2001) Hydroxyl radical-induced cell-wall loosening in vitro and in vivo: implications for the control of elongation growth. Plant J 28(6):679–688
Schopfer P, Liszkay A, Bechtold M, Frahry G, Wagner A (2002) Evidence that hydroxyl radicals mediate auxin-induced extension growth. Planta 214:821–828
Shapiguzov A, Vainonen JP, Wrzaczek M, Kangasjärvi J (2012) ROS-talk – how the apoplast, the chloroplast, and the nucleus get the message through. Front Plant Sci 3:1–9. https://doi.org/10.3389/fpls.2012.00292
Sharp RE, Poroyko V, Hejlek LG, Spollen WG, Springer GK, Bohnert HJ, Nguyen HT (2004) Root growth maintenance during water deficits: physiology to functional genomics. J Exp Bot 55:2343–2351
Silva-Navas J, Moreno-Risueno MA, Manzano C, Téllez-Robledo B, Navarro-NeilaS CV, Pollmann S, Gallego FJ, del Pozoa JC (2016) Flavonols mediate root phototropism and growth through regulation of proliferation-to-differentiation transition. Plant Cell 28:1372–1387. https://doi.org/10.1105/tpc.15.00857
Singh KL, Chaudhuri A, Kar RK (2015) Role of peroxidase activity and Ca2+ in axis growth during seed germination. Planta 242:997–1007
Singh KL, Mukherjee A, Kar RK (2017) Early axis growth during seed germination is gravitropic and mediated by ROS and calcium. J Plant Physiol 216:181–187
Singh KL, Chaudhuri A, Kar RK (2014) Superoxide and its metabolism during germination and axis growth of Vigna radiata (L.) Wilczek seeds. Plant Signal Behav 9(8):e29278
Tsukagoshi H (2016) Control of root growth and development by reactive oxygen species. Curr Opin Plant Biol 29:57–63. https://doi.org/10.1016/j.pbi.2015.09.008
Wada M, Kong S-G (2018) Actin-mediated movement of chloroplasts. J Cell Sci 131:1–8. https://doi.org/10.1242/jcs.210310
Woolley JT, Stoller EW (1978) Light penetration and light-induced seed germination in soil. Plant Physiol 61:597–600
Yokawa K, Baluska F (2016) The TOR complex: an emergency switch for root behavior. Plant Cell Physiol 57:14–18
Yokawa K, Kagenishi T, Kawano T, Mancuso S, Baluska F (2011) Illumination of Arabidopsis roots induces immediate burst of ROS production. Plant Signal Behav 6:1460–1464
Zhang J, Kirkham MB (1996) Lipid peroxidation in sorghum and sunflower seedlings as affected by ascorbic acid, benzoic acid, and propyl gallate. J Plant Physiol 149:489–493
Acknowledgements
One of the authors (AM) gratefully recognizes financial support for the present investigation from University Grants Commission (UGC), New Delhi, India as BSR Fellowship [vide letter F. No. 25-1/2014-15(BSR)/220/2009/(BSR)]. The authors also acknowledge the Departmental research facilities created under UGC-SAP and DST-FIST programs.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Communicated by V. P. Singh.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Dey, T., Das, S., Majumdar, A. et al. Apoplastic reactive oxygen species mediated escape growth of root during illumination in Vigna radiata (L.) Wilczek seedlings. Acta Physiol Plant 43, 145 (2021). https://doi.org/10.1007/s11738-021-03313-2
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11738-021-03313-2