Abstract
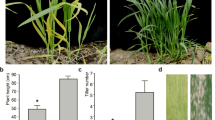
Leaf rolling is a common phenotypic trait and has recently gained more attention due to its involvement in photosynthetic efficiency and crop yield. An upward-curling leaf mutant (Bnucl1) was obtained from the rapeseed cultivar Zhongshuang 9 population (Brassica napus L.) via ethyl methanesulfonate mutagenesis. In this study, morphological and histological features, physiological characters, and inheritance of the Bnucl1 were investigated. The results indicated that leaf structure such as midvein, epidermal cells, and palisade mesophyll cells displayed remarkable differences in the mutant compared to wild type (WT). Pronounced variations in the chloroplast size and shape, starch grana between the mutant and WT leaves were observed, and the chloroplasts might be largely undifferentiated in the mutant. Moreover, the net photosynthetic rate, stomatal conductance, intercellular CO2 concentration, and transpiration rate were significantly decreased in the mutant. Likewise, chlorophyll a, chlorophyll b, total chlorophyll, and carotenoid contents were also significantly decreased in the mutant compared to WT. Enzyme activity of SOD, POD, and CAT was reduced; however, the content of H2O2 and MDA was increased in the mutant. Among the tested agronomic traits and leaf area, all the traits except siliques on the terminal raceme decreased in the mutant plants. Hence, the mutation has imposed remarkable impacts on the structure and function of leaves and agronomic traits. Genetic study indicated that this trait was monogenic and the allele for curling leaf was dominant. These results provide inroads for further evaluation to understand the mechanism of leaf development in B. napus.






Similar content being viewed by others
References
Alamin M, Zeng DD, Qin R, Sultana MH, Jin XL, Shi CH (2017) Characterization and fine mapping of SFL1, a gene controlling screw flag leaf in rice. Plant Mol Biol Rep 35:491–503
Alamin M, Zeng DD, Sultana MH, Qin R, Jin XL, Shi CH (2018) Photosynthesis, cellulose contents and ultrastructure changes of mutant rice leading to screw flag leaf. Plant Growth Regul 85:1–13
Bai L, Duan ZQ, Wang JM, An LZ, Zhao ZG, Chen KM (2008) Anatomical and chemical characteristics of a rolling leaf mutant of rice and its ecophysiological properties. Rice Sci 15(3):201–208
Bestwick CS, Brown IR, Mansfield JW (1998) Localized changes in peroxidase activity accompany hydrogen peroxide generation during the development of a nonhost hypersensitive reaction in Lettuce. Plant Physiol 118:1067–1078
Chen Q, Xie Q, Gao J, Wang W, Sun B, Liu B, Zhu H, Peng H, Zhao H, Liu C, Wang J, Zhang J, Zhang G, Zhang Z (2015) Characterization of Rolled and Erect Leaf 1 in regulating leave morphology in rice. J Exp Bot 66:6047–6058
Chen W, Wan S, Shen L, Zhou Y, Huang C, Chu P, Guan R (2018) Histological, physiological, and comparative proteomic analyses provide insights into leaf rolling in Brassica napus. J Proteome Res 17(5):1761–1772
Chitwood DH, Guo M, Nogueira FTS, Timmermans MCP (2007) Establishing leaf polarity: the role of small RNAs and positional signals in the shoot apex. Development 134:813–823
Clarke JM (1986) Effect of leaf rolling on leaf water loss in Triticum spp. Can J Plant Sci 66:885–891
Gao J (2006) Photosynthesis: determination of chlorophyll content. In: Gao J (ed) Experimental guidance for plant physiology. Higher Education Press, Beijing, pp 74–77
Giardi MT (1993) Phosphorylation and disassembly of the photosystem II core as an early stage of photoinhibition. Planta 190:107–113
Govaerts YM, Jacquemoud S, Verstraete MM, Ustin SL (1996) Three-dimensional radiation transfer modeling in a dicotyledon leaf. Appl Opt 35(33):6585–6598
Hayat S, Cheng Z, Ahmad H, Ali M, Chen X, Wang M (2016) Garlic, from remedy to stimulant: evaluation of antifungal potential reveals diversity in phytoalexin allicin content among garlic cultivars; allicin containing aqueous garlic extracts trigger antioxidants in cucumber. Front Plant Sci 7:1235
Horton P (2000) Prospects for crop improvement through the genetic manipulation of photosynthesis: morphological and biochemical aspects of light capture. J Exp Bot 51:475–485
Kadioglu A, Terzi R (2007) A dehydration avoidance mechanism: leaf rolling. Bot Rev 73(4):290–302
Kerstetter RA, Bollman K, Taylor RA, Bomblies K, Poethig RS (2001) KANADI regulates organ polarity in Arabidopsis. Nature 411:706–709
Kidner CA, Timmermans MCP (2010) Signaling sides: adaxial–abaxial patterning in leaves. Curr Top Dev Biol 91:141–168
Lang YZ, Zhang ZJ, Gu XY, Yang JC, Zhu QS (2004) Physiological and ecological effects of crimpy leaf character in rice (Oryza sativa L.) II. photosynthetic character, dry mass production and yield forming. Acta Agron Sin 30(9):883–887
Lee YK, Woo MO, Lee D, Lee G, Kim B, Koh HJ (2016) Identification of a novel candidate gene for rolled leaf in rice. Genes and Genomics 38:1077–1084
Li WQ, Zhang MJ, Gan PF, Qiao L, Yang SQ, Miao H, Wang GF, Zhang MM, Liu WT, Li HF, Shi CH, Chen KM (2017) CLD1/SRL1 modulates leaf rolling by affecting cell wall formation, epidermis integrity and water homeostasis in rice. Plant J 92:904–923
Li L, Staden JV, Jäger AK (1998) Effects of plant growth regulators on the antioxidant system in seedlings of two maize cultivars subjected to water stress. Plant Growth Regul 25:81–87
Liang J, Liu B, Wu J, Cheng F, Wang X (2016) Genetic variation and divergence of genes involved in leaf adaxial–abaxial polarity establishment in Brassica rapa. Front Plant Sci 7:94
Luo YZ, Zhao FM, Sang XC, Ling YH, Yang ZL, He GH (2009) Genetic analysis and gene mapping of a novel rolled-leaf mutant rl12(t) in rice. Acta Agron Sin 35(11):1967–1972
McConnell JR, Emery J, Eshed Y, Bao N, Bowman J, Barton MK (2001) Role of PHABULOSA and PHAVOLUTA in determining radial patterning in shoots. Nature 411(6838):709–713
Miller G, Shulaev V, Mittler R (2008) Reactive oxygen signaling and abiotic stress. Physiol Plant 133:481–489
Mittler R, Vanderauwera S, Gollery M, Breusegem FV (2004) Reactive oxygen gene network of plants. Trends Plant Sci 9:490–498
Moloi MJ, Westhuizen AJVD (2006) The reactive oxygen species are involved in resistance responses of wheat to the Russian wheat aphid. J Plant Physiol 163:1118–1125
Nogueira FTS, Madi S, Chitwood DH, Juarez MT, Timmermans MCP (2007) Two small regulatory RNAs establish opposing fates of a developmental axis. Genes Dev 21:750–755
Ohsumi A, Hamasaki A, Nakagawa H, Yoshida H, Shiraiwa T, Horie T (2007) A model explaining genotypic and ontogenetic variation of leaf photosynthetic rate in rice (Oryza sativa) based on leaf nitrogen content and stomatal conductance. Ann Bot 99:265–273
Pekker I, Alvarez JP, Eshed Y (2005) Auxin response factors mediate Arabidopsis organ asymmetry via modulation of KANADI activity. Plant Cell 17:2899–2910
Qu GP, Sun YY, Pang HX, Wu Q, Wang FL, Hu SW (2014) EMS mutagenesis and ALS-inhibitor herbicide-resistant mutants of Brassica napus L. Chin J Oil Crop Sci 36(1):025–031
Roach T, Krieger-liszkay A (2014) Regulation of photosynthetic electron transport and photoinhibition. Curr Protein Pept Sci 15(4):351–362
Saruhan N, Terzi R, Saglam A, Kadioglu A (2009) The relationship between leaf rolling and ascorbate-glutathione cycle enzymes in apoplastic and symplastic areas of Ctenanthe setosa subjected to drought stress. Biol Res 42:315–326
Saruhan N, Terzi R, Sağlam A, Kadioğlu A (2010) Scavenging of reactive oxygen species in apoplastic and symplastic areas of rolled leaves in Ctenanthe setosa under drought stress. Acta Biol Hung 61(3):282–298
Scarpeci TE, Zanor MI, Valle EM (2017) Estimation of stomatal aperture in Arabidopsis thaliana using silicone rubber imprints. Bio-protocol 7(12):e2347
Siegfried KR, Eshed Y, Baum SF, Otsuga D, Drews GN, Bowman JL (1999) Members of the YABBY gene family specify abaxial cell fate in Arabidopsis. Development 126:4117–4128
Teng S, Qian Q, Zeng D, Kunihiro Y, Fujimoto K, Huang D, Zhu L (2004) QTL analysis of leaf photosynthetic rate and related physiological traits in rice (Oryza sativa L.). Euphytica 135:1–7
Vos CHRD, Schat H, Waal MAMD, Vooijs R, Ernst WHO (1991) Increased resistance to copper-induced damage of the root cell plasmalemma in copper tolerant Silene cucubalus. Physiol Plant 82:523–528
Wang LF, Fu H, Ji YH (2012) Photosynthetic characterization of a rolled leaf mutant of rice (Oryza sativa L.). Afr J Biotechnol 11(26):6839–6846
Wang Y, Chen W, Chu P, Wan S, Yang M, Wang M, Guan R (2016) Mapping a major QTL responsible for dwarf architecture in Brassica napus using a single-nucleotide polymorphism marker approach. BMC Plant Biol 16:178
Wellburn AR (1994) The spectral determination of Chlorophylls a and b, as well as total carotenoids, using various solvents with spectrophotometers of different resolution. J Plant Physiol 144:307–313
Xiao Y, Tholen D, Zhu XG (2016) The influence of leaf anatomy on the internal light environment and photosynthetic electron transport rate: exploration with a new leaf ray tracing model. J Exp Bot 67:6021–6035
Xu DQ, Shen YG (1994) Progress on physiology of crop high production and high efficiency. Science Publishing Company, Beijing, pp 17–23
Yang EN, Yang ZJ, Zhang JF, Zou YC, Ren ZL (2011) Molecular cytogenetic characterization of a new leaf rolling triticale. Genet Mol Res 10(4):2953–2961
Ye W, Hu S, Wu L, Ge C, Cui Y, Chen P, Xu J, Dong G, Guo L, Qian Q (2017) Fine mapping a major QTL qFCC7L for chlorophyll content in rice (Oryza sativa L.) cv. PA64s. Plant Growth Regul 81:81–90
Zhang GH, Xu Q, Zhu XD, Qian Q, Xue HW (2009) SHALLOT-LIKE1 is a KANADI transcription factor that modulates rice leaf rolling by regulating leaf abaxial cell development. Plant Cell 21:719–735
Zhang J, Zhang H, Srivastava AK, Pan Y, Bai J, Fang J, Shi H, Zhu JK (2018) Knockdown of rice microRNA166 confers drought resistance by causing leaf rolling and altering stem xylem development. Plant Physiol 176:2082–2094
Zhu Q, Yu S, Chen G, Ke L, Pan D (2017) Analysis of the differential gene and protein expression profile of the rolled leaf mutant of transgenic rice (Oryza sativa L.). PLoS ONE 12(7):e0181378
Zou LP, Sun XH, Zhang ZG, Liu P, Wu JX, Tian CJ, Qiu JL, Lu TG (2011) Leaf rolling controlled by the homeodomain leucine zipper class IV gene Roc5 in Rice. Plant Physiol 156:1589–1602
Acknowledgements
This research work was supported by the National Key Research and Development Program of China (2018YFD0100603), Natural Science Foundation in Shaanxi Province of China (2018JQ3046), Key Research and Development Project in Shaanxi Province of China (2018ZDXM-NY-008), and Modern Crop Seed Industry Project of Shaanxi Province (20171010000004).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Communicated by B. Zheng.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Faisal, S., Guo, Y., Du, C. et al. Morphological, physiological and genetic analyses of an upward-curling leaf mutant in Brassica napus L.. Acta Physiol Plant 42, 46 (2020). https://doi.org/10.1007/s11738-020-3033-4
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11738-020-3033-4