Abstract
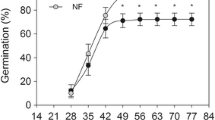
The distribution of woody species in flooded environments depends on the duration of stress as well as seed germination strategies and plant morphophysiological adaptations. Allophylus edulis is a tree that occurs in temporarily or permanently flooded areas in several countries of South America. In this paper, we evaluate seed germination, growth parameters, photosynthetic pigment contents and chlorophyll a fluorescence in young plants to understand the tolerance of the specie to flood events. The evaluations were performed in non-flooded (NFL) and flooded (FL) plants in a temporal scale that included short (up to 30 days) and long (up to 90 days) flood periods. A short flooding (15 days) may favor germination but maintaining stress for 60 days makes the seeds unviable. Although 71.4% of the FL plants survived up to 90 days of flooding, injuries such as chlorosis and foliar abscission appeared. An increase in stem height and diameter was only observed in NFL plants; whereas, FL plants showed a growth inhibition. At 90 days, NFL and FL plants presented total dry mass of 18.35 ± 1.57 g and 1.93 ± 0.62 g, respectively. The photosynthetic performance indexes indicated acclimatization of the plants on the third day of flooding, but the stress induced a progressive decline in the parameters, signaling damages to the photosystem II. Both seeds and young plants of A. edulis tolerate short periods of flooding, but prolonged floods make the seeds unfeasible and damages the photosynthetic apparatus, leading to death of the plants.





Similar content being viewed by others
References
Abreu DCA, Kuniyoshi YS, Nogueira AC, Medeiros A (2005) Caracterização morfológica de frutos, sementes e germinação de Allophylus edulis (St.-Hil.) Radlk. (Sapindaceae). Rev bras Sementes 27:59–66
Alvares CA, Stape JL, Sentelhas PC, Moraes G, Leonardo J, Sparovek G (2013) Köppen's climate classification map for Brazil. Meteorol Z 22:711–728
Alves JD, Zanandrea I, Deuner S, Goulart PFP, Souza KRD, Santos MO (2013) Antioxidative responses and morpho-anatomical adaptations to waterlogging in Sesbania virgata. Trees 27:717–728
Andrade ACSD, Ramos FN, Souza AFD, Loureiro MB, Bastos R (1999) Flooding effects in seedlings of Cytharexyllum myrianthum Cham. and Genipa americana L.: responses of two neotropical lowland tree species. Rev Bras Bot 22:281–285
Arnon DI (1949) Copper enzymes in isolated chloroplasts. Polyphrenol oxidase in Beta vulgaris. Plant Physiol 24:1–15
Bailey-Serres J, Voesenek LACJ (2008) Flooding stress: acclimations and genetic diversity. Annu Rev Plant Biol 59:313–339
Barbedo CJ, Marcos Filho J (1998) Tolerância à dessecação em sementes. Acta Bot Bras 12:145–164
Baskin CC, Baskin JM (2014) Seeds: ecology, biogeography and evolution of dormancy and germination. Academic Press, San Diego
Batista CUN, Medri ME, Bianchini E, Medri C, Pimenta JA (2008) Flood tolerance in Cecropia pachystachya Trec. (Cecropiaceae): ecophysiological and morpho-anatomical aspects. Acta Bot Bras 22:91–98
Benincasa MMP (2003) Análise de crescimento de plantas: noções básicas. Funep, Jaboticabal
Berjak P, Pammenter NW (2007) From Avicennia to Zizania: seed recalcitrance in perspective. Ann Bot 101:213–228
Bidalia A, Okram Z, Hanief M, Rao KS (2018) Assessment of tolerances in Mitragyna parvifolia (Roxb.) Korth and Syzygium cumini Keels. seedlings to waterlogging. Photosynthetica 56:707–717
Brasil, Ministério da Agricultura, Pecuária e Abastecimento (2009) Regras para análise de sementes. MAPA, Brasília
Buckeridge MS, Aidar MPM, Santos HP, Tiné MAS (2004) Acúmulo de reservas. In: Ferreira AG, Borghetti F (eds) Germinação: do básico ao aplicado. Artmed, Porto Alegre, pp 31–50
Colmer T, Voesenek L (2009) Flooding tolerance: suites of plant traits in variable environments. Funct Plant Biol 36:665–681
Conserva A, Camargo JLC, Santana DG, Piedade MTF (2018) Germinative behaviour of ten tree species in white-water floodplain forests in central Amazonia. Folia Geobot 53:89–101
Copertino MS, Creed JC, Lanari MO, Magalhães K, Barros K, Lana PC, Sordo L, Horta PA (2016) Seagrass and submerged aquatic vegetation (VAS) habitats off the coast of Brazil: state of knowledge, conservation and main threats. Braz J Oceanogr 64:53–80
da Paz AA, Ribeiro C, Azevedo AA, Lima ERD, Carmo FMDS (2017) Induced flooding as environmental filter for riparian tree species. Environ Exp Bot 139:31–38
Drew MC (1997) Oxygen deficiency and root metabolism: injury and acclimation under hypoxia and anoxia. Annu Rev Plant Physiol Plant Mol Biol 48:223–250
Duarte CI (2012) Influência da variação hidrológica e da luminosidade na composição e estrutura do componente herbáceo em uma floresta paludosa no extremo sul do Brasil. Dissertation, Universidade Federal do Rio Grande
Du K, Xu L, Wu H, Tu B, Zheng B (2012) Ecophysiological and morphological adaption to soil flooding of two poplar clones differing in flood-tolerance. Flora 207:96–106
Ferreira CS, Piedade MTF, Bonates LC (2006) Germinação de sementes e sobrevivência de plântulas de Himatanthus sucuuba (Spruce) Wood em resposta ao alagamento nas várzeas da Amazônia Central. Acta Amaz 36:413–418
Ferreira CS, Piedade MTF, Tiné MAS, Rossatto DR, Parolin P, Buckeridge MS (2009) The role of carbohydrates in seed germination and seedling establishment of Himatanthus sucuuba, an Amazonian tree with populations adapted to flooded and non-flooded conditions. Ann Bot 104:1111–1119
Gagetti BL, Piratelli AJ, Piña-Rodrigues FCM (2016) Fruit color preference by birds and applications to ecological restoration. Braz J Biol 76:955–966
Gattringer JP, Ludewig K, Harvolk-Schöning S, Donath TW, Otte A (2018) Interaction between depth and duration matters: flooding tolerance of 12 floodplain meadow species. Plant Ecol 219:973–984
Gravatt DA, Kirby CJ (1998) Patterns of photosynthesis and starch allocation in seedlings of four bottomland hardwood tree species subjected to flooding. Tree Physiol 18:411–417
Herrera A, Tezara W, Marín O, Rengifo E (2008) Stomatal and non-stomatal limitations of photosynthesis in trees of a tropical seasonally flooded forest. Physiol Plant 134:41–48
Joly CA, Crawford RMM (1982) Variation in tolerance and metabolic responses to flooding in some tropical trees. J Exp Bot 33:799–809
José AC, Silva EA, Davide AC (2007) Classificação fisiológica de sementes de cinco espécies arbóreas de mata ciliar quanto a tolerância à dessecação e ao armazenamento. Rev Bras Sementes 29:171–178. https://doi.org/10.1590/S0101-31222007000200023
Junior UMS, Gonçalves JFC, Strasser RJ, Fearnside PM (2015) Flooding of tropical forests in central Amazonia: what do the effects on the photosynthetic apparatus of trees tell us about species suitability for reforestation in extreme environments created by hydroelectric dams? Acta Physiol Plant 37:166
Junk WJ (2013) Current state of knowledge regarding South America wetlands and their future under global climate change. Aquat Sci 75:113–131
Kolb RM, Joly CA (2009) Flooding tolerance of Tabebuia cassinoides: metabolic, morphological and growth responses. Flora 204:528–535
Kozlowski TT (1997) Responses of woody plants to flooding and salinity. Tree Physiol. https://doi.org/10.1093/treephys/17.7.490
Kozlowski TT, Pallardy SG (2002) Acclimation and adaptive responses of woody plants to environmental stresses. Bot Rev 68:270–334
Larré CF, Fernando JA, Marini P, Bacarin MA, Peters JA (2013) Growth and chlorophyll a fluorescence in Erythrina crista-galli L. plants under flooding conditions. Acta Physiol Plant 35:1463–1471
Larré C, Leivas-Moraes C, Borella J, Amarante L, Deune S, Peters JA (2016) Antioxidant activity and fermentative metabolism in the plant Erythrina crista-galli L. under flood conditions. Semina Ciênc Agrár 37:567–580
Liu B, Rennenberg H, Kreuzwieser J (2015) Hypoxia affects nitrogen uptake and distribution in young poplar (Populus × canescens) trees. PLoS ONE 10(8):e0136579
Lucas CM, Mekdeçe F, Nascimento CM, Holanda ASS, Braga J, Dias S, Souza S, Rosa P, Suemitsu C (2012) Effects of short-term and prolonged saturation on seed germination of Amazonian floodplain forest species. Aquat Bot 99:49–55
Marques M, Joly CA (2000) Germinação e crescimento de Calophyllum brasiliense (Clusiaceae), uma espécie típica de florestas inundadas. Acta Bot Bras 14:113–120
Martinazzo EG, Perboni AT, Oliveira PV, Bianchi VJ, Bacarin MA (2013) Atividade fotossintética em plantas de ameixeira submetidas ao déficit hídrico e ao alagamento. Cienc Rural 43:35–41
Medina CL, Sanches MC, Tucci MLS, Sousa CA, Cuzzuol GRF, Joly CA (2009) Erythrina speciosa (Leguminosae-Papilionoideae) under soil water saturation: morphophysiological and growth responses. Ann Bot 104:671–680
Okamoto J, Joly CA (2000) Ecophysiology and respiratory metabolism during the germination of Inga sessilis (Vell.) Mart. (Mimosaceae) seeds subjected to hypoxia and anoxia. Braz J Bot 23:51–57
Oliveira VC, Joly CA (2010) Flooding tolerance of Calophyllum brasiliense Camb. (Clusiaceae): morphological, physiological and growth responses. Trees 24:185–193
Oliveira ACS, Martins GN, Silva RF, Vieira HD (2009) Testes de vigor em sementes baseados no desempenho de plântulas. InterSciencePlace 1:1–21
Oxborough K, Baker NR (1997) Resolving chlorophyll a fluorescence images of photosynthetic efficiency into photochemical and non-photochemical components-calculation of qP and Fv−/Fm−; without measuring Fo. Photosynth Res 54:135–142
Parolin P (2001) Senna reticulata, a pioneer tree from Amazonian várzea floodplains. Bot Rev 67:239–254
Parolin P, Wittman F (2010) Struggle in the flood: tree response to flooding stress in four tropical floodplain systems. AoB Plants. https://doi.org/10.1093/aobpla/plq003
Parolin P, Ferreira LV, Junk WJ (2003) Germination characteristics and establishment of trees from central Amazonian flood plains. Trop Ecol 44:157–169
Parolin P, De Simone O, Haase K, Waldhoff D, Rottenberger S, Kuhn U, Kesselmeier J, Kleiss B, Schmidt W, Piedade M et al (2004) Central Amazonian floodplain forests: tree adaptations in a pulsing system. Bot Rev 70:357–380
Peng Y, Zhou Z, Tong R, Hu X, Du K (2017) Anatomy and ultrastructure adaptations to soil flooding of two full-sib poplar clones differing in flood-tolerance. Flora 233:90–98
Pezeshki SR (2001) Wetland plant responses to soil flooding. Environ Exp Bot 46:299–312
Polacik KA, Maricle BR (2013) Effects of flooding on photosynthesis and root respiration in salt cedar (Tamarix ramosissima), an invasive riparian shrub. Environ Exp Bot 89:19–27
Reboita MS, Kruche N (2018) Normais Climatológicas Provisórias de 1991 a 2010 para Rio Grande. RS Rev bras meteorol 33:165–179
Rengifo E, Tezara W, Herrera A (2005) Water relations, chlorophyll a fluorescence, and contents of saccharides in tree species of a tropical forest in response to flood. Photosynthetica 43:203–210
Scarano FR (2002) Structure, function and floristic relationships of plant communities in stressful habitats marginal to the Brazilian Atlantic rainforest. Ann Bot 90:517–524
Scarano FR, Crawford RM (1992) Ontogeny and the concept of anoxia-tolerance: the case of the Amazonian leguminous tree Parkia pendula. J Trop Ecol 8:349–352
Scarano FR, Pereira TS, Rôças G (2003) Seed germination in Seed germination during floatation and seedling growth of Carapa guianensis, a tree from flood-prone forests of the Amazon. Plant Ecol 168:291–296
Silva AC, Van den Berg E, Higuchi P, Oliveira Filho AT (2007) Comparação florística de florestas inundáveis das regiões sudeste e sul do Brasil. Rev Bras Bot 30:257–269
Silva AC, Higuchi P, Van den Berg E, Nunes MH, Carvalho DA (2012) Florestas Inundáveis: Ecologia, florística e adaptações das espécies. Editora UFLA, Lavras
Smirnoff N (1993) The role of active oxygen in the response of plants to water deficit and desiccation. New Phytol 125:27–58
Strasser BJ, Strasser RJ (1995) Measuring fast fluorescence transients to address environmental questions: the JIP-test. In: Mathis P (ed) Photosynthesis: from light to biosphere. Kluwer Academic Publishers, Dordrecht, pp 977–980
Strasser RJ, Tsimilli-Michael M, Srivastava A (2004) Analysis of the chlorophyll a fluorescence transient. In: Papageorgiou GC, Govindjee G (eds) Chlorophyll a fluorescence: a signature of photosynthesis. Advances in photosynthesis and respiration series. Springer, Dordrecht, pp 321–362
Teixeira AP, Assis MA, Luize BG (2011) Vegetation and environment relationships in a neotropical swamp forest in southeastern Brazil (Itirapina, SP). Aquat Bot 94:17–23
Tewari S, Mishra A (2018) Flooding stress in plants and approaches to overcome. In: Ahmad P, Ahanger MA, Singh VP, Tripathi DK, Pravej Alam P, Alyemeni MN (eds) Plant metabolites and regulation under environmental stress. Academic Press, London, pp 356–366
Turchetto F, Araujo MM, Callegaro RM, Griebeler AM, Mezzomo JC, Berghetti ÁL, Rorato DG (2017) Phytosociology as a tool for forest restoration: a study case in the extreme South of Atlantic Forest Biome. Biodivers Conserv 26:1463–1480
Waechter JL, Jarenkow JA (1998) Composição e estrutura do componente arbóreo nas matas turfosas do Taim, Rio Grande do Sul. Biotemas 11:45–69
Wellburn AR (1994) The spectral determination of chlorophylls a and b, as well as total carotenoids, using various solvents with spectrophotometers of different resolution. J Plant Physiol 144:307–313
Wittmann AO, Piedade MTF, Parolin P, Wittmann F (2007) Germination of four low-varzea tree species in central Amazonia. Aquat Bot 86:197–203
Yu B, Zhao CY, Li J, Li JY, Peng G (2015) Morphological, physiological, and biochemical responses of Populus euphratica to soil flooding. Photosynthetica 53:110–117
Zar JH (1999) Biostatistical analysis. Prentice-Hall, Upper Saddle River
Zhang Q, Huber H, Beljaars SJ, Birnbaum D, Best S, Kroon H, Visser EJ (2017) Benefits of flooding-induced aquatic adventitious roots depend on the duration of submergence: linking plant performance to root functioning. Ann Bot 120:171–180
Zúñiga-Feest A, Bustos-Salazar A, Alves F, Martinez V, Smith-Ramírez C (2017) Physiological and morphological responses to permanent and intermittent waterlogging in seedlings of four evergreen trees of temperate swamp forests. Tree Physiol 37:779–789
Acknowledgements
We thank Ana Roschildt, Daniel Villanova, Mateus Negrini and Roger Oliveira their support in data collection. We also thank Ana S. Rolon, Fabiana Barbosa, Marianna Lanari for suggestions in previous version of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Communicated by P. Wojtaszek.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Duarte, C.I., Martinazzo, E.G., Bacarin, M.A. et al. Seed germination, growth and chlorophyll a fluorescence in young plants of Allophylus edulis in different periods of flooding. Acta Physiol Plant 42, 80 (2020). https://doi.org/10.1007/s11738-020-03063-7
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11738-020-03063-7