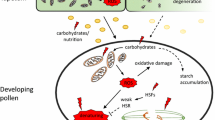
Abstract
Global warming and other climate change eventslead to a constant rise in ambient temperatures. Exposure to higher than optimal temperatures is negatively affecting sexual reproductive performance, leading to low seed set and a consequent yield reduction. Pollen is considered more heat stress sensitive than both vegetative tissues and the female gametophyte. But this sensitivity to heat stress is not uniform during pollen development and function. Pollen grains are more sensitive at the early stages of development (anther wall development, microsporogenesis and microgametogenesis) than pollen at later stages (pollen maturation and anther dehiscence) or during the progamic phase (pollen hydration, germination, growth and guidance of the pollen tube). Heat stress is thought to be sensed by four types of sensors including cyclic nucleotide-gated calcium channels (CNGC), unfolded protein response (UPR) in endoplasmic reticulum (ER) and cytosol and histone proteins in the nucleosome. Each sensor activates pollen heat stress response (HSR) through reprogramming of the transcriptome, proteome and metabolome. HSR restores cellular and metabolic homeostasis in the pollen and avoids damage elicited by heat stress. In the first part of this review, we have summarized current findings regarding the effect of heat stress on different stages of pollen development and performance. In the second part, the responses of pollen to heat stress are discussed. Finally, directions for future research on pollen HSR are discussed with a focus on breeding crops with improved thermo-tolerance, thus capable of withstanding the current context of global warming.


Similar content being viewed by others
References
Alghabari F, Lukac M, Jones HE, Gooding MJ (2014) Effect of Rht alleles on the tolerance of wheat grain set to high temperature and drought stress during booting and anthesis. J Agron Crop Sci 200:36–45
Angelos E, Ruberti C, Kim SJ, Brandizzi F (2017) Maintaining the factory: the roles of the unfolded protein response in cellular homeostasis in plants. Plant J 90:671–682
Aya K, Ueguchi-Tanaka M, Kondo M, Hamada K, Yano K, Nishimura M, Matsuoka M (2009) Gibberellin modulates anther development in rice via the transcriptional regulation of GAMYB. Plant Cell 21:1453–1472
Banerjee A, Roychoudhury A (2018) Interactions of Brassinosteroids with major phytohormones: antagonistic effects. J Plant Growth Regul Doi. https://doi.org/10.1007/s00344-018-9828-5
Bao Y, Howell SH (2017) The unfolded protein response supports plant development and defense as well as responses to abiotic stress. Front Plant Sci. https://doi.org/10.3389/fpls.2017.00344
Begcy K, Dresselhaus T (2018) Epigenetic responses to abiotic stresses during reproductive development in cereals. Plant Reprod. https://doi.org/10.1007/s00497-018-0343-4
Berenguer E, Barany I, Solis MT, Perez-Perez Y, Risueno MC et al (2017) Inhibition of histone H3K9 methylation by BIX-01294 promotes stress-induced microspore totipotency and enhances embryogenesis initiation. Front Plant Sci. https://doi.org/10.3389/fpls.2017.01161
Berni R, Luyckx M, Xuan X, Legay S, Sergeant K et al (2018) Reactive oxygen species and heavy metal stress in plants: impact on the cell wall and secondary metabolism. Environ Exp Bot. https://doi.org/10.1016/j.envexpbot.2018.10.017
Biancucci M, Mattioli R, Forlani G, Funck D, Costantino P et al (2015) Role of proline and GABA in sexual reproduction of angiosperms. Front Plant Sci. https://doi.org/10.3389/fpls.2015.00680
Boavida LC, Borges F, Becker JD, Feijo JA (2011) Whole genome analysis of gene expression reveals coordinated activation of signaling and metabolic pathways during pollen-pistil interactions in Arabidopsis. Plant Physiol 155:2066–2080
Boisson-Dernier A, Lituiev DS, Nestorova A, Franck CM, Thirugnanarajah S et al (2013) ANXUR receptor-like kinases coordinate cell wall integrity with growth at the pollen tube tip via NADPH oxidases. PLoS Biol. https://doi.org/10.1371/journal.pbio.1001719
Bokszczanin KL, Fragkostefanakis S (2013) Perspectives on deciphering mechanisms underlying plant heat stress response and thermotolerence. Front Plant Sci. https://doi.org/10.3389/fpls.2013.00315
Bokszczanin KL, Krezdorn N, Fragkostefanakis S, Rycak L, Chen Y et al (2015) Identification of novel small ncRNAs in pollen of tomato. BMC Genom. https://doi.org/10.1186/s12864-015-1901-x
Borg M, Berger F (2015) Chromatin remodeling during male gametophyte development. Plant J 83:177–188
Boursiac Y, Leran S, Corratge-Faillie C, Gojon A, Krouk G et al (2013) ABA transport and transporters. Trends Plant Sci 18:325–333
Cai W, Zhang D (2018) The role of receptor-like kinases in regulating plant male reproduction. Plant Reprod 31:77–87
Caldelari D, Wang G, Farmer EE, Dong X (2011) Arabidopsis lox3 lox4 double mutants are male sterile and defective in global proliferative arrest. Plant Mol Biol 75:25–33
Capovilla G, Schmid M, Pose D (2015) Control of flowering by ambient temperature. J Exp Bot 66:59–69
Cecchetti V, Altamura MM, Falasca G, Costantino P, Cardarelli M (2008) Auxin regulates Arabidopsis anther dehiscence, pollen maturation and filament elongation. Plant Cell 20:1760–1774
Cecchetti V, Celebrin D, Napoli N, Ghelli R, Brunetti P et al (2017) An auxin maximum in the middle layer controls stamen development and pollen maturation in Arabidopsis. New Phytol 2013:1194–1207
Chaturvedi P, Ischebeck T, Egelhofer V, Lichtscheidl I, Weckwerth W (2013) Cell-specific analysis of the tomato pollen proteome from pollen mother cell to mature pollen provides evidence for developmental priming. J Proteome Res 12:4892–4903
Chaturvedi P, Doerfler H, Jegadeesan S, Ghatak A, Pressman E et al (2015) Heat-treatment-responsive proteins in different developmental stages of tomato pollen detected by targeted mass accuracy precursor alignment (tMAPA). J Proteome Res 14:4463–4471
Chen Y, Muller F, Rieu I, Winter P (2016) Epigenetic events in plant male germ cell heat stress responses. Plant Reprod 29:21–29
Chen L, Yang D, Zhang Y, Wu L, Zhang Y et al (2018) Evidence for a specific and critical role of mitogen-activated protein kinase 20 uni-to-binucleate transition of microgametogenesis in tomato. New Phytol 219:176–194
Cheng H, Qin L, Lee S, Fu X, Richards DE et al (2004) Gibberellin regulates Arabidopsis floral development via suppression of DELLA protein function. Development 131:1055–1064
Chiang HH, Dandekar AM (1995) Regulation of proline accumulation in Arabidopsis thaliana (L.) Heynh during development and in response to desiccation. Plant Cell Environ 18:1280–1290
Chini A, Fonseca S, Fernandez G, Adie B, Chico JM et al (2007) The JAZ family of repressors is the missing link in jasmonate signaling. Nat 448:666–671
Ciampolini F, Shivanna KR, Cresti M (1990) High humidity and heat stress causes dissociation of endoplasmic reticulum in tobacco plants. Plant Biol 104:110–116
Clarke SM, Cristescu SM, Miersch O, Harren FJM, Wasternack C et al (2009) Jasmonates act with salicylic acid to confer basal thermotolerance in Arabidopsis thaliana. New Phytol 182:175–187
Colebrook EH, Thomas SG, Phillips AL, Hedden P (2014) The role of gibberellin signalling in plant responses to abiotic stress. J Exp Biol 217:67–75
Cortijo S, Charoensawan V, Brestovitsky A, Buning R, Ravarani C et al (2017) Transcriptional regulation of the ambient temperature response by H2A.Z nucleosomes and HSF1 transcription factors in Arabidopsis. Mol plant 10:1258–1273
Das K, Roychoudhury A (2014) Reactive oxygen species (ROS) and response of antioxidants as ROS-scavengers during environmental stress in plants. Front Environ Sci. https://doi.org/10.3389/fenvs.2014.00053
Datta R, Chamusco KC, Chourey PS (2002) Starch biosynthesis during pollen maturation is associated with altered patterns of gene expression in maize. Plant Physiol 130:1645–1656
Davis GL (1966) Systematic embryology of the angiosperms. Wiley, New York
De Storme N, Geelen D (2013) Sexual polyploidization in plants-cytological mechanisms and molecular regulation. New Phytol 198:670–684
De Storme N, Geelen D (2014) The impact of environmental stress on male reproductive development in plants: biological processes and molecular mechanisms. Plant Cell Environ 37:1–18
Deal RB, Kandaswamy MK, McKinney EC, Meagher RB (2005) The nuclear actin-related protein ARP6 is a pleiotropic developmental regulator required for the maintenance of FLOWERING LOCUS C expression and repression of flowering in Arabidopsis. Plant Cell 17:2633–2646
Deng Y, Humbert S, Liu JX, Srivastava R, Rothstein SJ et al (2011) Heat induces the splicing by IRE1 of a mRNA encoding a transcription factor involved in the unfolded protein response in Arabidopsis. Proc Natl Acad Sci USA 108:7247–7252
Deng Y, Srivastava R, Howell SH (2013) Endoplasmic reticulum (ER) stress response and its physiological roles in plants. Int J Mol Sci 14:8188–8212
Deng Y, Srivastava R, Quilichini TD, Dong H, Bao Y et al (2016) IRE1, a component of the unfolded protein response signaling pathway, protects pollen development in Arabidopsis from heat stress. Plant J 88:193–204
Devasirvatham V, Gaur PM, Mallikarjuna N, Raju TN, Trethowan RM et al (2013) Reproductive biology of chickpea response to heat stress in the field is associated with the performance in controlled environments. Field Crops Res 142:9–19
Ding Z, Wang B, Moreno I, Duplakova N, Simon S et al (2012) ER-localized auxin transporter PIN8 regulates auxin homeostasis and male gametophyte development in Arabidopsis. Nat Commun. https://doi.org/10.1038/ncomms1941
Distefano G, Hedhly A, Las Casas G, La Malfa S, Herrero M et al (2012) Male-female interactions and temperature variation affect pollen performance in Citrus. Sci Hortic 140:1–7
Djanaguiraman M, Prasad PVV, Boyle DL, Schapaugh WT (2013) Soybean pollen anatomy, viability and pod set under high temperature stress. J Agron Crop Sci 199:171–177
Djanaguiraman M, Prasad PVV, Murugan M, Perumal R, Reddy UK (2014) Physiological differences among sorghum (Sorghum bicolor L. Moench) genotypes under high temperature stress. Environ Exp Bot 100:43–54
Doucet J, Lee HK, Goring DR (2016) Pollen acceptance or rejection: a tale of two pathways. Trends Plant Sci 21:1058–1067
Dresselhaus T, Sprunck S, Wessel GM (2016) Fertilization mechanisms in flowering plants. Corr Biol 26:125–139
Driedonks N, Wolters-Arts M, Huber H, de Boer GJ, Vriezen W et al (2018) Exploring the natural variation for reproductive thermotolerance in wild tomato species. Euphytica. https://doi.org/10.1007/s10681-018-2150-2
Duan Q, Kita D, Johnson EA, Aggarwal M, Gates L, Wu HM, Cheung AY (2014) Reactive oxygen speciesmediate pollen tube rupture to release sperm for fertilization in Arabidospis. Nat Commun. https://doi.org/10.1038/ncomms4129
Endo M, Tsuchiya T, Hamada K, Kawamura S, Yano K et al (2009) High temperatures cause male sterility in rice plants with transcriptional alterations during pollen development. Plant Cell Physiol 50:1911–1922
Firon N, Shaked R, Peet MM, Pharr DM, Zamski E et al (2006) Pollen grains of heat tolerant tomato cultivars retains higher carbohydrate concentration under heat stress conditions. Sci Hortic 109:212–217
Firon N, Pressman E, Meir S, Khoury R, Altahan L (2012) Ethylene is involved in maintaining tomato (Solanum lycopersicum) pollen quality under heat-stress conditions. AoB Plants. https://doi.org/10.1093/aobpla/pls024
Foster J, Lee YH, Tegeder M (2008) Distinct expression of members of the LHT amino acid transporter family in flowers indicates specific roles in plant reproduction. Sex Plant Reprod 2:143–152
Fragkostefanakis S, Simm S, Paul P, Bublak D, Scharf KD et al (2015) Chaperone network composition in Solanum lycopersicum explored by transcriptome profiling and microarray meta-analysis. Plant Cell Environ 38:693–709
Fragkostefanakis S, Mesihovic A, Hu Y, Schleiff E (2016a) Unfolded protein response in pollen development and heat stress tolerance. Plant Reprod 29:81–91
Fragkostefanakis S, Mesihovic A, Simm S, Paupiere MJ, Hu Y et al (2016b) HsfA2 controls the activity of developmentally and stress-regulated heat stress protection mechanisms in tomato male reproductive tissues. Plant Physiol 170:2461–2477
Francis KE, Lam SY, Harrison BD, Bey AL, Berchowitz LE et al (2007) Pollen tetrad-based visual assay for meiotic recombination in Arabidopsis. Proc Natl Acad Sci USA 104:3913–3918
Frank G, Pressman E, Ophir R, Althan L, Shaked R et al (2009) Transcriptional profiling of maturing tomato (Solanum lycopersicum L.) microspores reveals the involvement of heat shock proteins, ROS scavengers, hormones, and sugars in the heat stress response. J Exp Bot 60:3891–3908
Gao F, Han X, Wu J, Zheng S, Shang Z et al (2012) A heat-activated calcium-permeable channel-Arabidopsis cyclic nucleotide-gated ion channel 6-is involved in heat shock responses. Plant J 70:1056–1069
Garcia CC, Nepi M, Pacini E (2017) It is a matter of timing: asynchrony during pollen development and its consequences on pollen performance in angiosperms-a review. Protoplasma 254:57–73
Gilroy S, Bialasek M, Suzuki N, Gorecka M, Devireddy A et al (2016) ROS, calcium and electric signals: key mediators of rapid systemic signaling in plants. Plant Physiol 171:1606–1615
Giorno F, Woters-Arts M, Grillo S, Scharf KD, Vriezen WH et al (2010) Developmental and heat stress-regulated expression of HsfA2 and small heat shock proteins in tomato anthers. J Exp Bot 61:452–462
Gogoi N, Farooq M, Barthakur S, Baroowa B, Paul S et al (2018) Thermal stress impacts on reproductive development and grain yield in grain legumes. J Plant Biol 61:265–291
Gomez JF, Talle B, Wilson ZA (2015) Anther and pollen development: a conserved developmental pathway. J Integr Plant Biol 57:876–891
Grove MD, Spencer GF, Rohwedder WK, Mandava N, Worley JF et al (1979) Brassinolide, a plant growth-promoting steroid isolated from Brassica napus pollen. Nature 281:216–217
Hafidh S, Fila J, Honys D (2016) Male gametophyte development and function in angiosperms: a general concept. Plant Reprod 29:31–51
Hahn A, Bublak D, Schleiff E, Scharf KD (2011) Crosstalk between Hsp90 and Hsp70 chaperones and heat stress transcription factors in tomato. Plant Cell 23:741–755
Hasegawa T, Fujimori S, Havlik P, Valin H, Bodirsky BL et al (2018) Risk of increased food insecurity under stringent global climate change mitigation policy. Nat Clim Change 8:699–703
Hatfield JL, Antle J, Garrett KA, Izaurralde RC, Mader T et al (2018) Indicators of climate change in agricultural systems. Clim Change. https://doi.org/10.1007/s10584-018-2222-2
Hayashi S, Wakasa Y, Takaiwa F (2013) Recent advances in understanding the control of secretory proteins by the unfolded protein response in plants. Int J Mol Sci 14:9396–93407
Hedhly A (2011) Sensitivity of flowering plant gametophytes to temperature fluctuations. Environ Exp Bot 74:9–16
Hedhly A, Hormaza JI, Herrero M (2004) Effect of temperature on pollen tube kinetics and dynamics in sweet cherry, Prunus avium (Rosaceae). Am J Bot 91:558–564
Higashiyama T, Takeuchi H (2015) The mechanism and key molecules involved in pollen tube guidance. Annu Rev Plant Biol 66:393–413
Higashiyama T, Yang WC (2017) Gametophytic pollen tube guidance: attractant peptides, gametic controls, and receptors. Plant Physiol 173:112–121
Hou Q, Ufer G, Bartels D (2016) Lipid signaling in plant responses to abiotic stress. Plant Cell Environ 39:1029–1048
Hu L, Liang W, Yin C, Cui X, Zong J et al (2011) Rice MADS3 regulates ROS homeostasis during late anther development. Plant Cell 23:515–533
Huang D, Wang S, Zhang B, Shang-Guan K, Shi Y et al (2015) A gibberellins-mediated DELLA-NAC signaling cascade regulates cellulose synthesis in rice. Plant cell 27:1681–1696
Huang S, Van Aken O, Schwarzlander M, Belt K, Millar A (2016) The roles of mitochondrial reactive oxygen species in cellular signaling and stress responses in plants. Plant Physiol 171:1551–1559
Hyun Y, Richter R, Coupland G (2017) Competence to flower: age controlled sensitivity to environmental cues. Plant Physiol 173:36–46
Ikeda M, Mistsuda N, Ohme-Takagi M (2011) Arabidopsis HsfB1 and HsfB2b act as repressors of the expression of heat-inducible Hsfs but positively regulate the acquired thermotolerance. Plant Physiol 157:1243–1254
Ingouff M, Berger F (2010) Histone3 variants in plants. Chromosoma 119:27–33
Irenaeus KST, Mitra SK (2014) Understanding the pollen and ovule characters and fruit set of fruit crops in relation to temperatures and genotype-a review. J Appl Bot Food Qual 87:157–167
Iwata Y, Fedoroff NV, Koizumi N (2008) Arabidopsis bZIP60 is a proteolysis-activated transcription factor involved in the endoplasmic reticulum stress response. Plant Cell 20:3107–3121
Jagadeesan S, Beery A, Altahan L, Meir S, Pressman E et al (2018) Ethylene production and signaling in tomato (Solanum lycopersicum) pollen grains is responsive to heat stress conditions. Plant Reprod. https://doi.org/10.1007/s00497-018-0339-0
Jagadish SVK, Muthurajan R, Oane R, Wheeler TR, Heuer S et al (2010) Physiological and proteomic approaches to address heat tolerance during anthesis in rice (Oryza sativa L.). J Exp Bot 61:143–156
Jain M, Prasad PV, Boote KJ, Hartwell AL Jr, Chourey PS (2007) Effects of season-long high temperature growth conditions on sugar-to-starch metabolism in developing microspores of grain sorghum (Sorghum bicolor L. Moench). Planta 227:67–79
Jiang J, Liu X, Liu C, Liu G, Li S et al (2017) Integrating omics and alternative splicing reveals insights into grape response to high temperature. Plant Physiol 173:1502–1518
Jiao J, Mizukami AG, Sankaranarayanan S, Yamguchi J, Itami K et al (2017) Structure-activity relation of AMOR sugar molecule that activates pollen tubes for ovule guidance. Plant Physiol 173:354–363
Jimenez-Quesada MJ, Traverso JA, Dios Alche JD (2016) NADPH oxidase-dependent superoxide production in plant reproductive tissues. Front Plant Sci. https://doi.org/10.3389/fpls.2016.00359
Jumrani K, Bhatia VS, Pandey GP (2018) Screening soyabean genotypes for high temperature tolerance by in vitro pollen germination, pollen tube length reproductive efficiency and seed yield. Ind J Plant Physiol 23:77–90
Kaur R, Bains TS, Bindumadhava H, Nayyar H (2015) Responses of mungbean (Vigna radiata L.) genotypes to heat stress:effects on reproductive biology, leaf function and yield traits. Sci Hortic 197:527–541
Kaushal N, Awasthi R, Gupta K, Gaur P, Siddique KHM et al (2013) Heat-stress-induced reproductive failures in chickpea (Cicer arietinum) is associated with impaired sucrose metabolism in leaves and anthers. Funct Plant Biol 40:1334–1349
Kaya H, Kakajima R, Iwano M, Kanaoka MM, Kimura S et al (2014) Ca2+-activated reactive oxygen species production by Arabidopsis RbohH and RbohJ is essential for proper pollen tube tip growth. Plant Cell 26:1069–1080
Kaya H, Iwano M, Takeda S, Kanaoka MM, Kimura S et al (2015) Apoplastic ROS production upon pollination by RbohH and RbohJ in Arabidopsis. Plant Signal Behav. https://doi.org/10.4161/15592324.2014.989050
Kazan K (2015) Diverse roles of jasmonates and ethylene in abiotic stress tolerance. Trends Plant Sci 20:219–229
Keller M, Hu Y, Mesihovic A, Fragkostefanakis S, Schleiff E et al (2017) Alternative splicing in tomato pollen in response to heat stress. DNA Res 24:205–217
Koski MH, Galloway LF (2017) Geographic variation in pollen color is associated with temperature stress. New Phytol 218:370–379
Kovaleva LV, Timofeeva GV, Zakharova EV, Voronkov AS, Rakitin VY (2011) Ethylene synthesis in petunia stigma tissues governs the growth of pollen tubes in progamic phase of fertilization. Rus J Plant Physiol 58:402–408
Kumar SV, Wigge PA (2010) H2A.Z-containing neucleosomes mediate the thermosensory response in Arabidopsis. Cell 140:136–147
Kumar RR, Goswami S, Gadpayle KA, Singh K, Sharma SK et al (2014) Ascorbic acid at pre-anthesis modulate the thermotolerance level of wheat (Triticum aestivum) pollen under heat stress. J Plant Biochem Biotechnol 23:293–306
Kurusu T, Kuchitsu K (2017) Autophagy, programmed cell death and reactive oxygen species in sexual reproduction in plants. J Plant Res 130:491–499
Kurusu T, Koyano T, Hanamata S, Kubo T, Noguchi Y et al (2014) OsATG7 is required for autophagy-dependent lipid metabolism in rice post meiotic anther development. Autophagy 10:878–888
Lachowiec J, Lemus T, Thomas JH, Murphy PJ, Nemhauser JL et al (2013) The protein chaperone HSP90 can facilitate the divergence of gene duplicates. Genet 193:1269–1277
Laloum T, Martin G, Duque P (2018) Alternative splicing control of abiotic stress responses. Trends Plant Sci 23:140–150
Lamke J, Brezenzinka K, Altmann S, Baurle I (2016) A hit-and-run heat shock factor governs sustained histone methylation and transcriptional stress memory. EMBO J 35:162–175
Larkindale J, Huang B (2005) Effects of abscisic acid, salicylic acid ethylene and hydrogen peroxide in thermotolerance and recovery for creeping bentgrass. Plant Growth Regul 47:17–28
Lassig R, Gutermuth T, Bey TD, Konrad KR, Romeis T (2014) Pollen tube NAD(P)H oxidases act as a speed control to dampen growth rate oscillations during polarized cell growth. Plant J 78:94–106
Lee SS, Jung WY, Park HJ, Lee A, Kwon SY et al (2018) Genome-wide analysis of alternative splicing in an inbred cabbage (Brassica oleracea L.) line ‘HO’ in response to heat stress. Curr Genom 19:12–20
Lehmann S, Gumy C, Blatter E, Boeffel S, Fricke W et al (2011) In planta function of compatible solute transporters of the AtProT family. J Exp Bot 62:787–796
Li Y, Ye Z, Nie Y, Zhang J, Wang GL et al (2015) Comparative phosphoproteome analysis of Magnaporthe oryzae-responsive proteins in susceptible and resistant rice cultivars. J Proteome 115:66–80
Li HJ, Meng JG, Yang WC (2018) Multilayered signaling pathways for pollen tube growth and guidance. Plant Reprod 31:31–41
Liu JX, Howell SH (2016) Managing the protein folding demands in the endoplasmic reticulum of plants. New Phytol 211:418–428
Liu HC, Liao HT, Charng YY (2011) The role of class A1 heat shock factors (HSFA1s) in response to heat and other stresses in Arabidopsis. Plant Cell Environ 34:738–751
Liu R, Loraine AE, Dickerson JA (2014) Comparisons of computational methods for differential alternative splicing detection using RNA-seq in plant systems. BMC Bioinform. https://doi.org/10.1186/s12859-014-0364-4
Lora J, Hormaza JI (2018) Pollen wall development in mango (Mangifera indica L., Anacardiaceae). Plant Reprod. https://doi.org/10.1007/s00497-018-0342-5
Ma H (2005) Molecular genetic analyses of microsporogenesis and microgametogenesis in flowering plants. Ann Rev Plant Biol 56:393–434
Matsui T, Omasa K (2002) Rice (Oryza sativa L.) cultivars tolerant to high temperature at flowering: anther characteristics. Ann Bot 89:683–687
Mattioli R, Biancucci M, Lonoce C, Costantino P, Trovato M (2012) Proline is required for male gametophyte development in Arabidopsis. BMC Plant Biol. https://doi.org/10.1186/1471-2229-12-236
Mazzeo MF, Cacace G, Lovieno P, Massarelli I, Grillo S et al (2018) Response mechanisms induced by exposure to high temperature in anthers from thermo-tolerant and thermo-sensitive tomato plants: a proteomic perspective. PLoS One. https://doi.org/10.1371/journal.pone.0201027
McCue AD, Panda K, Nuthikattu S et al (2015) ARGONAUTE 6 bridges transposable element mRNA-derived siRNAs to the establishment of DNA methylation. EMBO J 34:20–35
Mesihovic A, Iannacone R, Firon N, Fragkostefanakis S (2016) Heat stress regimes for the investigation of pollen thermotolerance in crop plants. Plant Reprod 29:93–105
Mignolet-Spruyt L, Xu E, Idanheimo N, Hoeberichts FA, Muhlenbock P et al (2016) Spreading the news: subcellular and organellar reactive oxygen species production and signalling. J Exp Bot 67:3831–3844
Mitsuda N, Seki M, Shinozaki K, Ohme-Takagi M (2005) The NAC transcription factors NST1 and NST2 of Arabidopsis regulate secondary wall thickenings and are required for anther dehiscence. Plant Cell 17:2993–3006
Mittler R (2017) ROS are good. Trends Plant Sci 22:11–19
Mittler R, Finka A, Goloubinoff P (2012) How do plants feel the heat? Trends Biochem Sci 37:118–125
Moon S, Kim SR, Zhao G, Yi J, Yoo Y et al (2013) Rice GLYCOSYLTRANSFERASE1 encodes a glycosyltransferase essential for pollen wall formation. Plant Physiol 16:663–675
Mroue S, Simeunovic A, Robert HS (2018) Auxin production as an integrator of environmental cues for developmental growth regulation. J Exp Bot 69:201–2012
Muller M, Munne-Bosch S (2015) Ethylene response factors: a key regulatory hub in hormone and stress signaling. Plant Physiol 169:32–41
Muller F, Rieu I (2016) Acclimation to high temperature during pollen development. Plant Reprod 29:107–118
Nagashima Y, Mishiba KI, Suzuki E, Shimada Y, Iwata Y et al (2011) Arabidopsis IRE1 catalyses unconventional splicing of bZIP60 mRNA to produce the active transcription factor. Sci Rep. https://doi.org/10.1038/srep00029
Nie WF, Wang MM, Xia XJ, Zhou YH, Shi K et al (2013) Silencing of tomato RBOH1 and MPK2 abolishes bressinosteroid-induced H2O2 generation and stress tolerance. Plant Cell Environ 36:789–803
Ohama N, Sato H, Shinozaki K, Yamaguchi-Shinozaki K (2017) Transcriptional regulatory network of plant heat stress response. Trends Plant Sci 22:53–65
Oshino T, Abiko M, Saito R, Ichiishi E, Endo M et al (2007) Premature progression of anther early developmental programs accompanied by comprehensive alterations in transcription during high-temperature injury in barley plants. Mol Genet Genom 278:31–42
Ozga JA, Kaur H, Savada RP, Reinecke DM (2016) Hormonal regulation of reproductive growth under normal and heat-stress conditions in legume and other model crop species. J Exp Bot 68:1885–1894
Pacini E, Guarnieri M, Nepi M (2006) Pollen carbohydrates and water content during development, presentation, and dispersal: a short review. Protoplasma 228:73–77
Palanivelu R, Preuss D (2000) Pollen tube targeting and axon guidance: parallels in tip growth mechanisms. Trends Cell Biol 10:517–524
Palanivelu R, Tsukamoto T (2011) Path finding in angiosperm reproduction: pollen tube guidance by pistils ensures successful double fertilization. Interdiscip Rev Dev Biol. https://doi.org/10.1002/wdev.6
Parish RW, Phan HA, Iacuone S, Li SF (2012) Tapetal development and abiotic stress: a center of vulnerability. Funct Plant Biol 39:553–559
Parrotta L, Faleri C, Cresti M, Cai G (2016) Heat stress affects the cytoskeleton and the delivery of sucrose synthase in tobacco pollen tubes. Planta 243:43–63
Paupiere MJ, van Heusden AW, Bovy AG (2014) The metabolic basis of pollen thermo-tolerance: perspectives for breeding. Metabolites 4:889–920
Paupiere MJ, van Haperen P, Rieu I, Visser RGF, Tikunov YM et al (2017) Screening for pollen tolerance to high temperatures in tomato. Euphytica. https://doi.org/10.1007/s10681-017-1927-z
Plackett ARG, Powers SJ, Fernandez-Garcia N, Urbanova T, Takebayashi Y et al (2012) Analysis of the developmental roles of the Arabidopsis gibberellin 20-oxidases demonstrates that GA20ox1, -2, and -3 are the dominant paralogs. Plant Cell 24:941–960
Plackett ARG, Ferguson AC, Powers SJ, Wanchoo-Kohli A, Phillips AL et al (2014) DELLA activity is required for successful pollen development in the columbia ecotype of Arabidopsis. New Phytol 201:825–836
Porch TG, Jahn M (2001) Effects of high-temperature stress on microsporogenesis in heat sensitive and heat-tolerant genotypes of Phaseolus vulgaris. Plant Cell Env 24:723–731
Port M, Tripp J, Zielinski D, Weber C, Heerklotz D et al (2004) Role of Hsp17.4-CII as coregulator and cytoplasmic retention factor of tomato heat stress transcription factor HsfA2. Plant Physiol 135:1457–1470
Potocky M, Jones MA, Bezvoda R, Smirnoff N, Zarsky V (2007) Reactive oxygen species produced by NADPH oxidase are involved in pollen tube growth. New Phytol 174:742–751
Pressman E, Shaked R, Shen S, Altahan L, Firon N (2012) Variations in carbohydrate content and sucrose-metabolizing enzymes in tomato (Solanum lycopersicum L.) stamen parts during pollen maturation. Am J Plant Sci 3:252–260
Quilichini TD, Douglas CJ, Samuels AL (2014) New views of tapetum ultrastructure and pollen exine development in Arabidopsis thaliana. Ann Bot 114:1189–1201
Renak D, Gibalova A, Solcova K, Honys D (2014) A new link between stress response and nucleolar function during pollen development in Arabidopsis mediated by AtREN1 protein. Plant Cell Environ 37:670–683
Rieu I, Twell D, Firon N (2017) Pollen development at high temperature: from acclimation to collapse. Plant Physiol 173:1967–1976
Sakata T, Oshino T, Miura S, Tomabechi M, Tsunaga Y et al (2010) Auxins reverse plant male sterility caused by high temperatures. Proc Natl Acad of Sci USA 107:8569–8574
Salinas-Grenet H, Herrera-Vasquez A, Parra S, Cortez A, Gutierrez L et al (2018) Modulation of auxin levels in pollen grains affects stamen development and anther dehiscence in Arabidopsis. Mol Sci https://doi.org/10.3390/ijms19092480
Sangu E, Tibazarwa FI, Nyomora A, Symonds RC (2015) Expression of genes for the biosynthesis of compatible solutes during pollen development under heat stress in tomato (Solanum lycopersicum). J Plant Physiol 178:10–16
Sangwan RS (1978) Change in the amino-acid content during male gametophyte formation of Datura metel in situ. Theor Appl Genet 52:221–225
Sanyal RP, Misra HS, Saini A (2018) Heat-stress priming and alternative splicing-linked memory. J Exp Bot 69:2431–2434
Sato S, Kamiyama M, Iwata T, Makita N, Furukawa H et al (2006) Moderate increase of mean daily temperature adversely affects fruit set of Lycopersicon esculentum by disrupting specific physiological processes in male reproductive development. Ann Bot 97:731–738
Scharf KD, Berberich T, Ebersberger I, Nover L (2012) The plant heat stress transcription factor (Hsf) family: structure, function and evolution. Biochim Biophys Acta 1819:104–119
Schijlen EG, de Vos CH, Martens S, Jonker HH, Rosin FM et al (2007) RNA interference silencing of chalcone synthase the first step in the flavonoid biosynthesis pathway, leads to parthenocarpic tomato fruits. Plant physiol 144:1520–1530
Sebastian JSV, Somayanda IM, Chiluwal A, Perumal R, Prasad PVV et al (2017) Resilience of pollen and post-flowering response in diverse sorghum genotypes exposed to heat stress under field conditions. Crop Sci 57:1658–1669
Selinski J, Scheibe R (2014) Pollen tube growth: where does the energy come from? Plant Signal Behav. https://doi.org/10.4161/15592324.2014.977200
Shang X, Cao Y, Ma L (2017) Alternative splicing in plant genes: a means of regulating the environmental fitness of plant. Int J Mol Sci. https://doi.org/10.3390/ijms18020432
Singh I, Shono M (2005) Physiological and molecular effects of 24-epibrassinolide, a brassinosteroid on thermotolerance of tomato. Plant Growth Regul. https://doi.org/10.1007/s10725-005-3252-0
Snider JL, Oosterhuis DM (2011) How does timing, duration and severity of heat stress influence pollen-pistil interactions in angiosperms? Plant Signal Behav 6:930–933
Song J, Nada K, Tachibana S (2001) The early increase of S-adenosylmethionine decarboxylase activity is essential for the normal germination and tube growth in tomato (Lycopersicon esculentum Mill.) pollen. Plant Sci 161:507–515
Song J, Nada K, Tachibana S (2002) Suppression of S-adenosylmethionine decarboxylase activity is a major cause for high-temperature inhibition of pollen germination and tube growth in tomato (Lycopersicon esculentum Mill.). Plant Cell Physiol 43:619–627
Song G, Wang M, Zeng B, Zhang J, Jiang C et al (2015) Anther response to high-temperature stress during development and pollen thermotolerance heterosis as revealed by pollen tube growth and in vitro pollen vigor analysis in upland cotton. Planta 241:1271–1285
Sura W, Kabza M, Karlowski WM, Bieluszewski T, Kus-Slowinska M et al (2017) Dual role of the histone variant H2A.Z in transcriptional regulation of stress-response genes. Plant Cell 29:791–807
Suzuki K, Takeda H, Tsukaguchi T, Egawa Y (2001) Ultrastructural study on degeneration of tapetum in anther of snap bean (Phaseolus vulgaris L.) under heat stress. Sex Plant Reprod 13:293–299
Suzuki N, Miller G, Morales J, Shulaev V, Torres MA et al (2011) Respiratory burst oxidases: the engines of ROS signaling. Curr Opin Plant Biol 14:691–699
Suzuki N, Bassil E, Hamilton JS, Inupakutaka MA, Zandalinas SI et al (2016) ABA is required for plant acclimation to a combination of salt and heat stress. PLoS One. https://doi.org/10.1371/journal.pone.0147625
Syed NH, Kalyana M, Marquez Y, Barta A, Brown JWS (2012) Alternative splicing in plants-coming of age. Trends Plant Sci 17:616–623
Takada K, Ishimaru K, Kamada H, Ezura H (2006) Anther-specific expression of mutated melon ethylene receptor gene Cm-ERS1/H70A affected tapetum degeneration and pollen grain production in transgenic tobacco plants. Plant Cell Reprod 25:936–941
Tang RS, Zheng JC, Jin ZQ, Zhang DD, Huang YH et al (2008) Possible correlation between high temperature-induced floret sterility and endogenous levels of IAA, GAs and ABA in rice (Oryza sativa L.). Plant Growth Regul 54:37–43
Tunc-Ozdemir M, Rato C, Brown E, Rogers S, Mooneyham A et al (2013a) Cyclic nucleotide gated channels 7 and 8 are essential for male reproductive fertility. PLoS One. https://doi.org/10.1371/journal.pone.0055277
Tunc-Ozdemir M, Tang C, Ishka MR, Brown E, Groves NR et al (2013b) A cyclic nucleotide-gated channel (CNGC16) in pollen is critical for stress tolerance in pollen reproductive development. Plant Physiol 161:1010–1020
Vabulas RM, Raychaudhuri S, Hayer-Hartl M, Hartl FU (2017) Protein folding in the cytoplasm and the heat shock response. Perspect Biol. https://doi.org/10.1101/cshperspect.a004390
van Dijk K, Ding Y, Malkaram S, Riethoven JJ, Liu R et al (2010) Dynamic changes in genome-wide histone H3 lysine 4 methylation patterns in response to dehydration stress in Arabidopsis thaliana. BMC Plant Biol. https://doi.org/10.1186/1471-2229-10-238
van den Heuvel KJPT, Barendse GWM, Wullems GJ (2001) Effect of gibberellic acid on cell division and cell elongation in anthers of the gibberellin deficient gib-1 mutant of tomato. Plant Biol 3:124–131
Verbruggen N, Hermans C (2008) Proline accumulation in plants: a review. Amino Acids 35:753–759
Verma V, Ravindran P, Kumar PP (2016) Plant hormone-mediate regulation of stress responses. BMC Plant Biol. https://doi.org/10.1186/s12870-016-0771-y
Vogler F, Konrad SSA, Sprunck S (2015) Knockin’ on pollen’s door: live cell imaging of early polarization events in germinating Arabidopsis pollen. Front Plant Sci. https://doi.org/10.3389/fpls.2015.00246
Wahid A, Gelani S, Ashraf M, Foolad M (2007) Heat tolerance in plants: an overview. Environ Exp Bot 61:199–223
Wang L, Ruan YL (2016) Critical roles of vacuolar invertase in floral organ development and male and female fertilities are revealed through characterization of GhVIN1-RNAi cotton plants. Plant Physiol 171:405–423
Wang Y, Ren F, Zhang X (2014) Spatial and temporal variations of regional high temperature events in China. Int J Climatol 34:3054–3065
Wang J, Li D, Shang F, Kang X (2017) High temperature-induced production of unreduced pollen and its cytological effects in Populous. Sci Rep. https://doi.org/10.1038/s41598-017-05661-x
Wei M, Song M, Fan S, Yu S (2013) Transcriptomic analysis of differentially expressed genes during anther development in genetic male sterile and wild type cotton by digital gene-expression profiling. BMC Genomics. https://doi.org/10.1186/1471-2164-14-97
Wilson ZA, Song J, Taylor B, Yang C (2011) The final split: the regulation of anther dehiscence. J Exp Bot 62:1633–1649
Wu G, Wang X, Li X, Kamiya Y, Otegui MS et al (2011) Methylation of a phosphatase specifies dephosphorylation and degradation of activated brassinosteroid receptors. Sci Signal. https://doi.org/10.1126/scisignal.2001258
Xia XJ, Zhou YH, Shi K, Zhou J, Foyer CH et al (2015) Interplay between reactive oxygen species and hormones in the control of plant development and stress tolerance. J Exp Bot 66:2839–2856
Xie HT, Wan ZY, Li S, Zhang Y (2014) Spatio-temporal production of reactive oxygen species by NADPH oxidase is critical for tapetal programmed cell death and pollen development in Arabidopsis. Plant Cell 26:2007–2023
Xu J, Ding Z, Vizcay-Barrena G, Shi J, Liang W et al (2014) ABORTED MICROSPORES acts as a master regulator of pollen wall formation in Arabidopsis. Plant Cell 26:1544–1556
Xu D, Shi J, Rautengarten C, Yang L, Qian X et al (2017) Defective pollen wall 2 (DPW2) encodes an Acyl transferase required for rice pollen development. Plant Physiol 173:240–255
Yang CJ, Zhang C, Lu YN, Jin JQ, Wang XL (2011) The mechanisms of brassinosteroids’ action: from signal transduction to plant development. Mol Plant 4:588–600
Ye Q, Zhu W, Li L, Zhang S, Yin Y et al (2010) Brassinosteroids control male fertility by regulating the expression of key genes involved in Arabidopsis anther and pollen development. Proc Natl Acad Sci USA 107:6100–6105
Yi J, Moon S, Lee YS, Zhu L, Liang W et al (2016) Defective tapetum cell death 1 (DTC1) regulates ROS levels by binding to metallothionein during tapetum degeneration. Plant Physiol 170:1611–1623
Yu SX, Feng QN, Xie HT, Li S, Zhang Y (2017) Reactive oxygen species mediate tapetal programmed cell death in tobacco and tomato. BMC Plant Biol. https://doi.org/10.1186/s12870-017-1025-3
Yuan L, Liu X, Luo M, Yang S, Wu K (2013) Involvement of histone modifications in plant abiotic stress responses. J Integr Plant Biol 55:892–901
Zafra A, Rodriguez-Garcia MI, Alche JD (2010) Cellular localization of ROS and NO in olive reproductive tissues during flower development. BMC Plant Biol. https://doi.org/10.1186/1471-2229-10-36
Zhang D, Yang L (2014) Specification of tapetum and microsporocyte cells within the anther. Curr Opin Plant Biol 17:49–55
Zhang C, Li G, Chen T, Feng B, Fu W et al (2018) Heat stress induces spikelet sterility in rice at anthesis through inhibition of pollen tube elongation interfering with auxin homeostasis in pollinated pistils. Rice. https://doi.org/10.1186/s12284-018-0206-5
Zhao LN, Shen LK, Zhang WZ, Zhang W, Wang Y et al (2013) Ca2+- dependent protein kinase 11 and 24 modulate the activity of the inward rectifying K+ channels in Arabidopsis pollen tubes. Plant Cell 25:649–661
Zhou L, Lan W, Jiang Y, Fang W, Luan S (2014) A calcium-dependent protein kinase interacts with and activates a calcium channel to regulate pollen tube growth. Mol Plant 7:369–376
Funding
Funding was provided by University Grants Commission [Grant No. F.NO. 41-451/2012 (SR)].
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by M. Capuana.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Raja, M.M., Vijayalakshmi, G., Naik, M.L. et al. Pollen development and function under heat stress: from effects to responses. Acta Physiol Plant 41, 47 (2019). https://doi.org/10.1007/s11738-019-2835-8
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11738-019-2835-8