Abstract
Rehmannia glutinosa Libosch., a valuable medicinal plant, was successfully propagated in vitro using shoot tip explants. Shoot multiplication was performed in glass tubes and in a nutrient sprinkle bioreactor. A mixture of 0.1 mg L−1 indole-3-acetic acid (IAA) and 1.0 mg L−1 of 6-benzylaminopurine in Murashige and Skoog (MS) agar-solidified medium proved the best combination for multiple shoot induction, yielding 8.2 shoots per explant after 4 weeks of culture in glass tubes. The number of shoots increased to 21 per explant when the same combination of growth regulators was used in a nutrient sprinkle bioreactor. The shoots rooted with a frequency of 93 % after 6 weeks of culture on MS agar medium supplemented with IAA (0.1 mg L−1) before being acclimatized in the greenhouse. The antioxidant activities of methanolic extracts from the leaves and roots of the in vitro-regenerated plants of R. glutinosa cultivated in the greenhouse were evaluated using four in vitro assays: scavenging of free radicals (DPPH and ABTS), transition metal reduction and total antioxidant activity phosphomolybdenum test. In all cases, the methanolic extract from leaves demonstrated better antioxidant activity than those taken from roots. A strong correlation was found between total phenolic and flavonoid content, and the antioxidant capacity of the studied extracts.
Similar content being viewed by others
Introduction
Rehmannia glutinosa Libosch. (Orobanchaceae) is a perennial plant whose tubular flowers have awarded it the common name Chinese foxglove (Park et al. 2009). The plant naturally occurs in Japan, China and Korea. The fresh, dried or steamed roots of R. glutinosa (Rehmanniae radix––Di Huang) are officially listed in the Chinese Pharmacopoeia (2000) and are widely used in Chinese traditional medicine as anti-anemic, anti-pyretic, anti-inflammatory, hypoglycemic, anti-hypertensive and anti-senescence agents (Kitagawa et al. 1991; Zhang et al. 2008) and are effective at regulating immunity and preventing tumors (Zhao et al. 2007). The manifold properties of the plant roots have been attributed to the presence of various secondary metabolites, mainly iridoid glucosides (catalpol and aucubin) and phenylpropanoids (verbascoside and isoverbascoside) as well as polysaccharides and phenolic acids (Anh et al. 2003; Chung et al. 2006; Zhang et al. 2008). However, little is known about the presence of flavonoids in the plants; only one study by Chung et al. (2006) reports the production of small amounts of hesperidin, naringin and myricetin and traces of quercetin, naringenin and kaempferol.
Annual demand of fresh Rehmannia root is 2 × 107 kg, which sells at a price of 1,200 USD/kg (Zhou et al. 2010). However, owing to its difficulty of cultivation (poor seed viability, virus infection, low and unpredictable yields and slow plant growth rate) (Park et al. 2009; Xu and Davey 1983; Zhao et al. 2007), low distribution and increasing demand access to this species is very limited and there is a need to develop an effective propagation method using in vitro culture techniques.
Some studies describe the micropropagation of the plant species by adventitious shoot formation on callus derived from leaves (Matsumoto et al. 1989; Xuesen et al. 2002; Zhenchen et al. 2004 and Park et al. 2009) or roots (Jeong et al. 2002). Clonal micropropagation of this species from shoot tips was reported by Shoyama et al. (1983); Paek et al. (1995) and Hatano et al. (1997). However, no R. glutinosa micropropagation protocol has yet evaluated the role played by the type and concentration of cytokinins in axillary shoot multiplication.
In addition, studies of antioxidant activity and the content of total phenolic compounds of R. glutinosa have so far been limited to the roots of field-growing plants (Kirby and Schmidt 1997; Cai et al. 2004; Wong et al. 2006; Li et al. 2008).
The aim of the present report was to study the morphogenic responses of shoot tip explants of R. glutinosa to various cytokinins (TDZ, BAP and kinetin). This is the first study to describe the use of a liquid culture in a nutrient sprinkle bioreactor as a mean for obtaining large-scale shoot production. The proliferated shoots were then rooted, and the regenerated plantlets acclimatized ex vitro. The antioxidant activity of the in vitro micropropagated plants was evaluated by in vitro assays performed on leaves and roots, and the results were compared to those of plants of the same age propagated in vivo from seeds. For a better description of the antioxidant profile of the plants, the total contents of phenolic compounds and flavonoids were determined by colorimetry.
Materials and methods
Plant material
The Rehmannia glutinosa Libosch. shoot tips were derived from 4-week-old in vitro-cultured shoots initiated from seeds obtained in 2006 from the Medicinal Plant Garden of the Medical University in Wrocław (Poland). The seeds were surface-sterilized with 2 % (v/v) sodium hypochlorite solution for 15–20 min and rinsed three times with sterile, distilled water. They were then aseptically germinated in glass tubes (Ø 20 mm), purchased from SLINAP (Łódź, Poland) containing 25 mL of agar-solidified 0.7 % w/v MS (Murashige and Skoog 1962) medium without growth regulators supplemented with sucrose (3 % w/v). The agar was supplied by Sigma Aldrich. After 4 weeks 80 % of seeds germinated. Then shoot tips were transferred into glass tubes containing agar-solidified MS medium supplemented with BAP (1.0 mg L−1) and IAA (0.1 mg L−1) and subcultured every 4 weeks into fresh medium to multiply plant material for further experiments.
Effect of cytokinins on shoot multiplication
For the experiment, 3- to 5-mm-long shoot tips from shoots grown in vitro for 15–17 subcultures were excised and transferred individually into glass tubes containing agar MS medium supplemented with IAA (0.1 mg L−1) and different concentrations of kinetin, BAP (0.5; 1 or 2 mg L−1) or TDZ (0.2; 0.5 or 1.0 mg L−1). The pH of all media was adjusted to 5.6–5.8 by 0.1 N NaOH. For all experiments growth regulators were added prior to autoclaving. All media were autoclaved at 0.1 MPa, 121 °C, for 17 min. The cultures were kept in the growth chamber at 26 ± 2 °C under 16 h light/8 h dark photoperiod (cool-white fluorescent light; 40 μmol m−2 s−1). These incubation conditions were maintained throughout the study.
After 4 weeks of culture, the percentage of explants forming axillary shoots and their average number per explant were recorded. Additionally, the percentage of explants producing a callus and the percentage of hyperhydrous shoots were also estimated. The experiments were repeated three times, in three subsequent subcultures. A total of 30 shoot tips were used for each experiment at each cytokinin concentration. The results are presented in Table 1.
Shoot multiplication in the nutrient sprinkle bioreactor
To increase the culture scale, the R. glutinosa shoots were allowed to proliferate in a 5.0 L (working volume 3.0 L) nutrient sprinkle bioreactor. The bioreactor consisted of a growth container and a control box made of glass and stainless steel using a spraying nozzle made of polypropylene. The upper part of the container was the growing area for the shoots and the bottom comprised a 1.0 L reservoir of nutrient medium (liquid MS medium supplemented with 1.0 mg L−1 BAP and 0.1 mg L−1 IAA). Nutrient medium was administered to the shoot culture by a peristaltic pump through a nozzle situated at the bottom of the growth container, the cultures being supported on a stainless steel wire mesh situated 18 cm above the bottom. The circulation rate of the medium was 60 mL per each 40 s delivery, with 3.0 min breaks between each delivery.
The bioreactor was inoculated with twenty 3–5 mm shoot tips derived from the shoots after 16th–18th subcultures. Each shoot tip contained one apical bud with 2 leaf primordia.
An average fresh weight (FW) of inoculum was 0.4 ± 0.01 g and the average dry weight (DW) was 0.024 ± 0.005 g.
After 4 weeks of culture, the multiple shoots cultured in the bioreactor were harvested for growth analysis. The total number of shoots produced over the culture period in the bioreactor was measured and the multiplication rate, that is the number of shoots multiplied from a single shoot tip, was calculated. The total shoot biomass (g L−1) as average fresh weight (FW) and average dry weight (DW) after each bioreactor run were determined. The results are presented in Table 2.
Shoot rooting and plant acclimatization
For the rooting experiments, 4-week-old axillary shoots multiplied in liquid medium in the bioreactor as well as in the glass tubes on agar-solidified MS medium with IAA (0.1 mg L−1) and BAP (1.0 mg L−1) were used. They were separated and transferred individually into tubes containing 25 mL of solid MS medium without growth regulators or with auxin (IAA or IBA) at a concentration of 0.1 mg L−1. After 4 and 6 weeks of culture, the percentage of shoots forming roots, the average number of roots per shoot and their average length (mm) were recorded. Rooting experiments were repeated three times in three subsequent subcultures separately for shoots multiplied in the tubes as well as in the bioreactor. A total of 30 shoots were used for each experiment. The data is presented in Fig. 1.
Rooting efficiency of R. glutinosa shoots after 4 (a, c, e) and 6 weeks (b, d, f) of culture. Shoots were multiplied on solid MS medium with BAP (1 mg L−1) and IAA (0.1 mg L−1) in glass tubes (light bars) and in liquid medium in bioreactor (dark bars). Shoots were rooted on MS medium with or without an auxin. Data is given as mean ± SE (n = 30). The means followed by the same letters within each diagram do not differ statistically according to the Kruskal–Wallis test (P ≤ 0.05)
Well-rooted 6-week-old plantlets with two or three pairs of leaves, growing on MS agar rooting media (see above) were washed in water to remove agar from the roots and transferred into pots (Ø 10 cm) containing a sterilized mixture of soil, sand and peat (4:3:3 v/v/v). The plantlets were covered with glass to ensure high humidity. The glass covers were gradually opened during the acclimatization period and removed completely after 14 days. In total, 29 plantlets rooted on hormone-free MS medium, 30 plantlets rooted on MS medium with IAA (0.1 mg L−1) and 30 plantlets rooted on MS medium with IBA (0.1 mg L−1) were taken for acclimatization. The potted plantlets were maintained inside a growth chamber at 26 ± 2 °C under a 16 h light regime (cool-white fluorescent light; 40 μmol m−2 s−1), for 4 weeks. After this time, pots with acclimatized plants were returned to the greenhouse and the survival rate, being the percentage of all potted plantlets still alive, was recorded 4 weeks later.
Antioxidant activities
Preparation of extracts
The leaves and roots of 10-week-old in vitro-regenerated plants, as well as the leaves and roots of the in vivo seed-derived R. glutinosa plants (named as in vivo-derived plants) which had been growing in the soil for 10 weeks were lyophilized, powdered and extracted with methanol (40 mL). The extraction procedure was performed according to Sesterhenn et al. (2007) with slight modifications described earlier (Piątczak et al. 2012). Extraction yields (% w/w) were calculated by the following formula: weight of the dried extract (g)/weight of the original sample (g) × 100 %. The extract yields were as follows: for extracts derived from in vitro-regenerated plants 26.4 % (leaves) and 54.7 % (roots) and for in vivo-derived plant extracts: 58.8 % (leaves) and 52.7 % (roots).
Total phenolic content
Total phenolic contents were measured using the method described by Singleton and Rossi (1965). The extract (400 µL) was mixed with 2 mL of Folin–Ciocalteu reagent (POCh, Poland) diluted tenfold, and 1.6 mL of 7.5 % sodium carbonate. The absorbance was determined after 30 min of incubation at room temperature at 765 nm. A spectrophotometer Ray Leigh UV-1601 produced by Beijing Reyleigh Corp. China was used throughout the study. The results were expressed as gallic acid mg equivalents per gram of dry extract (GAE mg g−1). The calibration curve consisted of prepared gallic acid solutions at concentrations of 1–400 mg L−1.
Total flavonoid content
The colorimetric evaluation of flavonoid content was performed according to Lamaison and Carnat (1990). Quantification was performed with using a standard quercetin calibration curve. The results were expressed as quercetin mg equivalents per gram of dry extract. The calibration curve consisted of prepared quercetin solutions at concentrations ranging from 1 to 100 mg L−1.
Total antioxidant activity
Total antioxidant activity was estimated by the phosphomolybdenum assay (P-Mo) as described by Prieto et al. (1999). Total antioxidant activity was expressed as ascorbic acid (AA) g equivalents per gram of dry extract.
DPPH radical scavenging assay
The radical scavenging activity of plant extracts against 1,1-diphenyl-2-picrylhydrazyl free radical (DPPH·) (Sigma Aldrich) was determined spectrophotometrically as described by Weremczuk-Jeżyna et al. (2013). The results were expressed as EC50 (μg mL−1): the concentration of sample at which 50 % of maximum scavenging activity was recorded.
ABTS radical scavenging assay
The antioxidant activity was also determined using the ABTS· radical cation decolourisation test according to Re et al. (1999). An ABTS stock solution was prepared by a mixture of 7 mM 2,2′-azinobis-(3 ethylbenzothiazoline-6-sulfonate)––ABTS (Sigma Aldrich) with 2.45 mM potassium persulfate (Sigma Aldrich). This mixture was kept in the dark at room temperature for 12–16 h. Then, 2 mL ABTS solution was mixed with 2 mL of methanol to give an absorbance of the negative control 0.7 ± 0.05 at 734 nm. The assay was performed by adding 2 mL of extract at concentrations of 2.0; 20.0; 100.0; 200.0; 500.0 and 1,000.0 µg mL−1 to 2 mL of ABTS solution. The absorbance was measured after 10 min at 734 nm. The results were expressed as EC50 (μg mL−1): the concentration of sample at which 50 % of maximum scavenging activity was recorded. Additionally, the results were calculated as Trolox μmol equivalents per gram of dry extract using a 1–20 μmol calibration curve of Trolox solutions.
FRAP (ferric reducing antioxidant power) assay
The FRAP assay was determined according to Pulido et al. (2000) with modifications. The antioxidant activity was determined against a standard of known FRAP value, ferrous sulphate, calculated from a calibration curve with concentrations from 0 to 2000 µM. The antioxidant activity was expressed in μmol Fe(II) g–1 of dry extract.
The antioxidant abilities of the R. glutinosa methanolic extracts, as determined by DPPH, ABTS, FRAP and P-Mo assays, are presented in Table 3. In all four assays, α-tocopherol was used as standard.
Statistical analysis
All data are presented as the average ± standard error (SE). The results presented in Table 1 were analyzed by Mann–Whitney’s U test, and the results presented in Figs. 1, 2 and Table 3 were analyzed using Kruskal–Wallis test. The level of significance was set at P ≤ 0.05. STATISTICA 10.0 (STATSoft, Poland) software was used for all calculations.
Total phenolic (a) and flavonoid contents (b) of methanolic extracts of R. glutinosa leaves and roots Plant material derived from: 1 roots and 2 leaves from in vitro-derived plants; 3 roots and 4 leaves from seed-derived plants. All plants were 10 weeks old. Data is given as mean ± SE (n = 3). The means followed by the same letters within each diagram do not differ statistically according to the Kruskal–Wallis test (P ≤ 0.05)
Each antioxidant assay as well as phenolic and flavonoid contents was repeated twice, and each sample was repeated three times. EC50 and correlation coefficients between antioxidant assays and the total phenolic and flavonoid contents were calculated using MS-Excel software.
Results and discussion
Effect of cytokinins on shoot multiplication
The effects of various concentrations of cytokinins: BAP and kinetin at concentrations 0.5, 1.0 and 2.0 mg mL−1 and TDZ at concentrations 0.2, 0.5 and 1.0 mg mL−1 on the proliferation of R. glutinosa shoots from shoot tip explants are presented in Table 1. The experiment was carried out in glass tubes and cytokinin was added to MS agar-solidified medium supplemented with IAA (0.1 mg L−1). The concentrations of growth regulators were selected on the base of preliminary studies made in our laboratory as well as on the literature data (Shoyama et al. 1983). Shoot multiplication was achieved within 4 weeks by axillary bud formation from existing meristems. Additionally, all media used in the study caused callus formation at the base of explants, which produced adventitious buds. The buds, however, did not develop into shoots and were not suitable for further multiplication or rooting. Mark and Simpson (1994) suggest that the phenomenon of callus formation could be associated with increased auxin accumulation at the basal parts of explants.
It was found that TDZ induced a greater number of shoots per explant than other cytokinins. The highest number of shoots (12.25 ± 3.99 per explant) was induced on medium containing 1.0 mg L−1 (4.54 μM) TDZ. However, explants grown on media with the cytokinin displayed necrosis of the meristematic area and only a very low percentage of explants responded: 13.3 % at 1.0 mg L−1 TDZ (Table 1). In addition, dependent on the cytokinin concentration, 63–80 % of the regenerated shoots exhibited hyperhydricity. TDZ has been demonstrated to have such a disadvantageous effect on axillary shoot proliferation in several other plant species: Cardiospermum halicacabum (Jahan and Anis 2009) or Codonopsis pilosula (Słupski et al. 2011), especially at higher concentrations (0.5–10.0 μM). On the other hand, TDZ at concentrations 0.5–4.0 mg L−1 was used by Park et al. (2009) for R. glutinosa adventitious shoot regeneration from leaf-derived callus; in this case, the percentage of explants showing shoot induction and number of shoots per explants responded was relatively low: maximum 67 and 3.8 %, respectively.
The addition of BAP or kinetin to shoot multiplication MS medium, containing 0.1 mg L−1 IAA, revealed better quality of shoots, but reduced number of shoots per explant. When shoot tips were incubated with kinetin, the frequency of explants forming shoots ranged between 45 and 63 %, dependent on cytokinin level, but the average number of shoots per explant was low: 3 per explant. It was found that in the presence of kinetin, complete plantlets with well-developed root systems can be obtained after 4 weeks of culture in shoot multiplication medium, removing the need for in vitro root induction before acclimatization. A similar response at 0.5 and 3.0 mg L−1 the cytokinin has been documented in Melissa officinalis by Meszaros et al. (1999). However, in the case of R. glutinosa, the average frequency of explants responding by plantlet formation on medium containing kinetin was only about 20 %.
Good R. glutinosa shoot production was achieved when 1.0 mg L−1 BAP (4.45 μM) was added to MS medium supplemented with 0.1 mg L−1 IAA. On the medium, 80 % of explants produced an average of 8 axillary shoots from a single explant within 4 weeks (Fig. 3a). Shoyama et al. (1983) report that the greatest level of shoot multiplication on R. glutinosa var. purpurea Makino, 20 axillary shoots per explant were obtained using a concentration of 5.0 mg L−1 BAP. However, in the present study, BAP concentrations lower or higher than 1.0 mg L−1, (i.e. 0.5 and 2.0 mg L−1) were found to decrease the percentage of explants forming shoots, as well as the average number of shoots per explant, and favored callus growth at the base of explants (Table 1). The differences in results obtained by us and those reported by Shoyama et al. (1983) may be attributed to the different source of explants (root segments). In the short communication of Shoyama et al. (1983) there is no information about shoot quality which had been induced. In our study, based on the frequency of shoot induction, number of shoots per explant and quality of regenerated shoots, i.e., the percentage of hyperhydricity, MS medium with 1 mg L−1 BAP and 0.1 mg L−1 IAA were chosen for further R. glutinosa shoot multiplication in tubes and in a bioreactor.
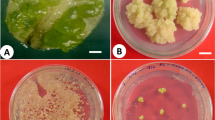
Micropropagation of R. glutinosa. Multiple shoots cultured for 4 weeks on solid MS medium with BAP (1 mg L−1) and IAA (0.1 mg L−1) in glass tubes (a); shoots in the nutrient sprinkle bioreactor (b); and after removing from the bioreactor (c); the plantlet rooted on MS medium with IAA (0.1 mg L−1) after 6 weeks of growth (d); an acclimatized plant after 8 weeks of growth in the pot in a greenhouse (e). Bars = 1 cm
Shoot multiplication in the sprinkle bioreactor
In the experiment, 20 single explants (shoot tips) of R. glutinosa were inoculated into a 5 L sprinkle bioreactor. The explants were placed on a steel net and sprinkled, as described in the “Materials and methods” section, with liquid MS medium supplemented with IAA (0.1 mg L−1) and BAP (1.0 mg L−1) dispersed into droplets. Under these conditions, shoots grew homogenously in all directions, such that the bioreactor was full after 4 weeks (Fig. 3b). An average 423 ± 14.5 shoots over the culture period was obtained in the bioreactor (Fig. 3c), giving a multiplication rate of 21, estimated by the average number of shoots per single explant (Table 2). This value was three times higher than that of the shoots multiplied in the tubes on solid medium of the same composition. As shown in Table 2, the final fresh and dry weights of shoot biomass in the bioreactor were 188 g L−1 (0.4 g per shoot) and 11.4 g L−1 (0.03 g per shoot), respectively. Studies performed on several other plant species cultured in liquid media, in various bioreactor types also have shown that shoot growth and their multiplication were more efficient than those achieved on solid media (Konstas and Kintzios 2003). However, a serious problem was the hyperhydricity of shoots cultured in liquid media (Shaik et al. 2010). This was not observed in the case of R. glutinosa. The shoots multiplied in the sprinkle bioreactor were found to have normal morphology without signs of hyperhydricity and apex necrosis. This is the first known report to use a bioreactor for R. glutinosa shoot multiplication. Earlier, the sprinkle bioreactor was successfully used in our laboratory for Centaurium erythraea shoot proliferation (Piątczak et al. 2005). The data obtained in the present study indicated that application of the sprinkle bioreactor for mass multiplication of R. glutinosa was very effective.
Shoot rooting and plant acclimatization
The individual shoots of R. glutinosa with normal morphology were multiplied on solid MS media, supplemented with IAA (0.1 mg L−1) and BAP (1.0 mg L−1), in glass tubes, and in liquid culture in the bioreactor. They were then taken for rooting and transferred to MS agar-solidified medium with or without the addition of auxin (IAA or IBA) at a concentration of 0.1 mg L−1. Root induction was observed after 2 weeks but the complete root development took 6 weeks (Fig. 3d). The results presented in Fig. 1 indicated that a higher frequency of root formation with significant difference at P ≤ 0.05, higher average number of roots per shoot and greater root length were recorded for shoots taken from the bioreactor, maybe due to the better quality of shoots in the bioreactor, especially lack of hyperhydricity. The highest rooting response was achieved when shoots from the bioreactor were rooted on MS medium with 0.1 mg L−1 IAA, and when the culture period was prolonged to 6 weeks (Fig. 3d). Under these conditions, 93 % of shoots developed an average of 5.3 ± 0.3 roots with a mean length of 21 mm (Figs. 1, 3). The values were lower in the presence of 0.1 mg L−1 IBA, but the differences between media containing IBA and IAA were not significant. R. glutinosa shoots formed roots also on MS medium without auxin, but with a much lower frequency. After 6 weeks of culture on the medium, 65–80 % of shoots formed an average of 3–5 roots (Fig. 1). These results are difficult to compare with the data from literature, because the other authors did not give the rooting results of R. glutinosa shoots derived from shoot tips. All plantlets generated from shoots multiplied in the bioreactor and rooted for 6 weeks on medium with or without auxin survived after an 8-week acclimatization period (Fig. 3e). For plantlets derived from shoots multiplied in tubes on solid medium, the survival rate was 93 % for shoots rooted on medium containing auxin and only 86 % for shoots rooted on MS medium without auxin. Only Park et al. (2009) described the percent of survived plants of R. glutinosa in pots during acclimatization period. It was lower (73 %) than that achieved in our study, but the plants were regenerated from adventitious shoots on leaf explants. The rhizogenesis process plays an important role during the acclimatization of regenerated plants, and a well-developed root system is one of the factors guaranteeing a high survival rate. Better acclimatization ability of the R. glutinosa plantlets originated from shoots grown in the bioreactor is probably due to the fact that the plantlets produced more roots, which were also longer (Fig. 1). Sudha et al. (2005) report that the highest survival rate was demonstrated by Decelepis arayalpathra in vitro-regenerated plants which created the highest number of roots during the rooting stage (7–9 per shoot).
Antioxidant activities and total content of phenolics and flavonoids
Antioxidant activities of methanolic extracts from leaves and roots of R. glutinosa plants were evaluated. The plants were regenerated in vitro or derived from seeds. To fully characterize their antioxidant properties, four in vitro tests based on different reaction mechanisms were used in this study. The free radical scavenging activity of the extracts tested was evaluated by DPPH and ABTS assays, while the FRAP assay was employed to measure the capacity to reduce iron ions (Fe3+ to Fe2+). Using the phosphomolybdenum assay (P-Mo), the total antioxidant activity of extracts was also analyzed as mg ascorbic acid equivalents per gram dry weight. The results are summarized in Table 3.
In all tests, methanolic extracts from the aerial parts of R. glutinosa plants revealed a stronger antioxidant activity than extracts from the roots of the plants. The results obtained in the DPPH and ABTS tests showed that the methanol extracts of the leaves of seed-derived plants exhibited slightly lower values of EC50 (60.2 μg mL−1 for DPPH and 37.9 μg mL−1 for ABTS), which means higher free radical scavenging capacity than those achieved for methanol extracts of leaves from in vitro-derived plants (EC50 = 80.9 and 40.3 μg mL−1, respectively). A similar tendency was observed in the FRAP test (Table 3). In the P-Mo assay, extracts of the leaves from both the in vitro- and seed-derived R. glutinosa plants showed similar antioxidant activity, not exceeding 0.5 g ascorbate equivalents g−1 DW (Table 3). The complexity of the mechanisms involved in antioxidant activity can be seen in the differences between the results of various antioxidant assays observed by many research groups (e.g., Wong et al. 2006; Li et al. 2008).
As the present study is the first report on antioxidant activity of extracts from aerial parts of R. glutinosa plants, the results obtained herein cannot be directly compared with those of other studies. Previous studies only examined the antioxidant activity (i.e., the ability to scavenge free radicals and to reduce ferric ions) of root extracts from R. glutinosa plants growing naturally. For example, Wong et al. (2006) screened the antioxidant activity of methanolic extracts from 30 Chinese medicinal plants, including R. glutinosa roots using the FRAP test and found that the most active plants had FRAP values above 500 (µmol Fe(II) g−1 DW). In comparison, the R. glutinosa leaf extracts tested in the present study were among the most highly active, with FRAP values ranging from 523 to 734 µmol Fe(II) g−1 DW (Table 3). In the same paper, Wong et al. (2006) report that the ability of R. glutinosa root extract to reduce ferric ions was 162 µmol Fe(II) g−1 DW. This value is similar to that observed in our study for the root extract of seed-derived plants (184 µmol Fe(II) g−1 DW), whereas the root extracts from the in vitro-regenerated plant were less potent (80 µmol Fe(II) g−1 DW). As these observed differences might be attributed to the differences in level of phenolic compounds in the analyzed extracts, colorimetric determination of total phenolic and flavonoid content was performed; this represents a simple and rapid assay of the whole complex of compounds possessing a large capacity to neutralize free radicals to trap single oxygen and to reduce and chelate transition metal ions (Matkowski 2008).
As shown in Fig. 2, the highest phenolic contents were found in extracts of leaves from R. glutinosa. It was indicated that total phenolic accumulation was lower in leaves of in vitro-derived plants (58.7 GAE mg g−1 DW) than in leaves of plants derived from seeds (76.3 GAE mg g−1 DW). On the other hand, the extract from leaves of in vitro-regenerated plants showed the highest level of flavonoids (19.2 quercetin mg g−1 DW).
The value was ~20 % higher than those of leaves from seed-propagated plants of R. glutinosa (Fig. 2b). It is known that exogenous supply of different plant growth regulators during in vitro propagation stage was considerably influenced the production of phenolic and flavonoids in tissue culture-derived plants. It results in the difference of antioxidant properties between in vitro and conventionally propagated plants (Ncube et al. 2011; Aremu et al. 2013). However, the nature of carry-over effect of cytokinin on the level of secondary metabolites in plants after the acclimatization stage remains unclear and unpredictable.
Low phenolic and flavonoid contents were found in extracts of R. glutinosa roots (Fig. 2a, b). It is known that the predominant compounds in R. glutinosa roots are iridoid glucosides, which are a group of compounds with poor antioxidant properties (Es-Safi et al. 2007; Wu et al. 2009). Table 4 showed significant linear correlations between total phenolic and flavonoid levels and the antioxidant capacities of the R. glutinosa extracts tested. This high correlation may confirm that the groups of compounds are responsible for the antioxidant activity of R. glutinosa extracts.
Conclusion
The present study demonstrates that R. glutinosa can be successfully propagated in vitro using shoot tip explants. Of the cytokinins tested, BAP was more effective for shoot induction than TDZ and kinetin, and the highest shoot proliferation was achieved on MS medium supplemented with IAA (0.1 mg L−1) and BAP (1.0 mg L−1). Liquid culture in the sprinkle bioreactor also appears to be a promising way for large-scale production of R. glutinosa plants. Rooting and ex vitro acclimatization of the plants are nonproblematic. The production takes 8–10 weeks from shoot multiplication to the transplantation of plants to the soil, during which time it is possible to obtain almost 400 plants from 20 shoot tips. Our results indicate that there are significant differences in antioxidant properties, as well as phenolic and flavonoid content within R. glutinosa plants depending on the organ tested. In addition, leaf extracts may be considered as a source of phenolic compounds with measurable antioxidant activity, while the roots showed lower antioxidant activity.
Author contribution
This research paper was accomplished with the collaboration of all authors. E. Piątczak obtained shoot cultures, performed micropropagation experiments in tubes and in the bioreactor, took photographic documentation and described the results. I. Grzegorczyk-Karolak performed antioxidant assays. H. Wysokińska was responsible for verification of the paper.
Abbreviations
- BAP:
-
6-Benzylaminopurine
- DW:
-
Dry weight
- FW:
-
Fresh weight
- IAA:
-
Indole-3-acetic acid
- IBA:
-
Indole-3-butyric acid
- MS:
-
Murashige and Skoog medium (Murashige and Skoog 1962)
- SE:
-
Standard error
- TDZ:
-
1-Phenyl-3-(1,2,3-thiadiazol-5-yl) urea thidiazuron
References
Anh NTH, Sung TV, Fronke K, Wessjohann LA (2003) Phytochemical studies of Rehmannia glutinosa rhizomes. Pharmazie 58:593–595
Aremu AO, Gruz J, Šubrtová M, Szűčová L, Doležal K, Bairu MW, Finnie JF, van Staden J (2013) Antioxidant and phenolic acid profiles of tissue cultured and acclimatized Merwilla plumbea plantlets in relation to the applied cytokinins. J Plant Physiol 170:1303–1308. doi:10.1007/s11738-012-1027-6
Cai Y, Luo Q, Sun M, Corke H (2004) Antioxidant activity and phenolic compounds of 112 traditional Chinese medicinal plants associated with anticancer. Life Sci 74:2157–2184. doi:10.1016/j.lfs.2003.09.047
Chung IM, Kim JJ, Lim JD, Yu ChY, Kim SH, Hahn SJ (2006) Comparison of resveratrol, SOD activity, phenolic compounds and free amino acids in Rehmannia glutinosa under temperature and water stress. Environ Exp Bot 56:44–53. doi:10.1016/j.envexpbot.2005.01.001
Es-Safi N-E, Kollmann A, Khlifi S, Ducrot P-H (2007) Antioxidative effect of compounds isolated from Globularia alypum L. structure–activity relationship. LWT 40:1246–1252. doi:10.1016/j.lwt.2006.08.019
Hatano M, Nakai R, Kawanishi F, Kedo K, Shoyama Y (1997) Genetic diagnosis of Rehmannia species micropropagated by tip tissue culture and an F1 hybrid by RAPD analysis. Plant Breed 116:589–591
Jahan AA, Anis M (2009) In vitro rapid multiplication and propagation of Cardiospermum halicacabum L. through axillary bud culture. Acta Physiol Plant 31:133–138. doi:10.1007/s11738-008-0211-1
Jeong JH, Yu KW, Chakrabarty D, Kim SJ, Peak KY (2002) In vitro regeneration and plantlet formation from adventitious roots of R. glutinosa Liboschits. Propag Ornam Plants 2:19–23
Kirby A, Schmidt RJ (1997) The antioxidant activity of Chinese herbs for eczema and of placebo herbs: I. J Ethnopharmacol 56:103–108
Kitagawa I, Fukuda Y, Taniyama T, Yoshikawa M (1991) Chemical studies on crude drug processing. VII. On the constituents of Rehmanniae radix. (1): absolute stereostructures of rehmaglutins A, B and D isolated from Chinese Rehmanniae Radix, the dried root of R. glutinosa Libosch. Chem Pharm Bull 39:1171–1176
Konstas J, Kintzios S (2003) Developing a scale-up system for the micropropagation of cucumber (Cucumis sativus L.): the effect of growth retardants, liquid culture and vessel size. Plant Cell Rep 21:538–548. doi:10.1007/s00299-002-0566-5
Lamaison JL, Carnat A (1990) Content of principal flawonoids of the flowers and leaves of Crataegus monogyna Jacq. and Crataegus laeviagata (Poiret) D.C. Rosaceae. Pharm Acta Helv 65:315–320
Li HB, Wong CC, Cheng KW, Chen F (2008) Antioxidant properties in vitro and total phenolic contents in methanol extracts from medicinal plants. LWT 41:385–390. doi:10.1016/j.lwt.2007.03.011
Mark TR, Simpson SE (1994) Factor affecting shoots development in apically dominant Acer cultivars in vitro. J Hortic Sci 69:543–551
Matkowski A (2008) Plant in vitro culture for the production of antioxidants––a review. Biotechnol Adv 26(548):560. doi:10.1016/j.biotechadv.2008.07.001
Matsumoto M, Shoyama Y, Nishioka I, Irino N (1989) Constituents of regenerated and shoot cultured root tissue of R. glutinosa. Phytochemistry 28:2331–2332
Meszaros A, Bellon A, Pinter E, Horvath G (1999) Micropropagation of lemon balm. Plant Cell Tiss Org Cult 57:149–152
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassay with tobacco culture. Physiol Plant 15:473–497
Ncube B, Ngunge VNP, Finnie JF, Van Staden J (2011) A comparative study of the antimicrobial and phytochemical properties between outdoor grown and micropropagated Tulbaghia violacea Harv. plants. J Ethnopharmacol 134:775–780
Paek KY, Yu KJ, Park SI, Sung NS, Park CH (1995) Micropropagation of R. glutinosa as medicinal plant by shoot tip and root segment culture. ISHS international symposium on medicinal and aromatic plants. Acta Hortic 390:120–123
Park SU, Kim YK, Lee SY (2009) Improved in vitro plant regeneration and micropropagation of R. glutinosa L. J Med Plants Res 3:31–34
Pharmacopoeia Commission of the People’s Republic of China (2000) The pharmacopoeia of the People’s Republic of China, vol 1. Chemical Industry Publishing House, Beijing, p 94
Piątczak E, Chmiel A, Wysokińska H (2005) Mist trickling bioreactor for Centaurium erythraea Rafn growth of shoots and production of secoiridoids. Biotechnol Lett 27:721–724. doi:10.1007/s10529-005-5189-9
Piątczak E, Królicka A, Wielanek M, Wysokińska H (2012) Hairy root cultures of R. glutinosa and the production of iridoid and phenylethanoid glycosides. Acta Physiol Plant 34:2215–2224. doi:10.1007/s11738-012-1022-y
Prieto P, Pineda M, Aguilar M (1999) Spectrophotometric quantitation of antioxidant capacity through the formation of a phosphomolybdenum complex: specific application to the determination of vitamin E. Anal Biochem 269:337–341
Pulido R, Bravo L, Saura-Calixto F (2000) Antioxidant activity of dietary polyphenols as determined by a modified ferric reducing antioxidant power assay. J Agricult Food Chem 48:3396–3402. doi:10.1021/jf9913458
Re RP, Pellegrini A, Pannala A, Yang M, Rice-Evans C (1999) Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic Biol Med 26:1231–1237
Sesterhenn K, Distl M, Wink M (2007) Occurrence of iridoid glycosides in in vitro cultures and intact plants of Scrophularia nodosa L. Plant Cell Rep 26:365–371. doi:10.1007/s00299-006-0233-3
Shaik S, Dewir YH, Singh N, Nicholas A (2010) Micropropagation and bioreactor studies of the medicinally important plant Lessertia (Sutherlandia) frutescens. South Afr J Bot 76:180–186. doi:10.1016/j.sajb.2009.10.005
Shoyama Y, Nagano M, Nishioka I (1983) Clonal multiplication of R. glutinosa. Planta Med 48:124–128
Singleton V, Rossi JA (1965) Colorimetry of total phenolics with phosphomolybdic––phosphotungstic acid reagents. Am J Enol Viticult 16:144–158
Słupski W, Tubek B, Matkowski A (2011) Micropropagation of Codonopsis pilosula (Franch.) Nannf. by axillary shoot multiplication. Acta Biol Crac Series Botanica 53/2:87–93. doi:10.2478/v10182-011-0031-2
Sudha CG, Krishnan PN, Pushpangadan P, Seeni S (2005) In vitro propagation of Decelepis arayalpathra, a critically endangered ethnomedicinal plant. In Vitro Cell Dev Biol Plant 41:648–654. doi:10.1079/IVP2005652
Weremczuk-Jeżyna I, Grzegorczyk-Karolak I, Frydrych B, Królicka A, Wysokińska H (2013) Hairy roots of Dracocephalum moldavica: Rosmarinic acid content and antioxidant potential. Acta Physiol Plant 35:2095–2103. doi:10.1007/s11738-013-1244-7
Wong CC, Li HB, Cheng KW, Chen F (2006) A systematic survey of antioxidant activity of 30 Chinese medicinal plants using the ferric reducing antioxidant power assay. Food Chem 97:705–711. doi:10.1016/j.foodchem.2005.05.049
Wu M, Wu P, Liu M, Xie H, Jiang Y, Wei X (2009) Iridoids in Gentiana loureirii. Phytochemistry 70:746–750. doi:10.1016/j.phytochem.2009.03.018
Xu XH, Davey MR (1983) Shoot regeneration from mesophyll protoplasts and leaf explants of R. glutinosa. Plant Cell Rep 2:55–57
Xuesen W, Delan H, Xiaoju M, Xianen L, Junhua Z (2002) Multiplication of virus-free seedlings of R. glutinosa. Zhongcaoyao 33:452–455
Zhang RX, Li MX, Jia ZP (2008) Rehmannia glutinosa: review of botany, chemistry and pharmacology. J Ethnopharm 117:199–214. doi:10.1016/j.jep.2008.02.018
Zhao H, Tan J, Qi Ch (2007) Photosynthesis of R. glutinosa subjected to drought stress is enhanced by choline chloride through alleviating lipid peroxidation and increasing proline accumulation. Plant Growth Reg 51:255–262. doi:10.1007/s10725-007-9167-1
Zhenchen Z, Qi Q, Xiulan J, Yongijang W (2004) The techniques and application of virus-free R. glutinosa. Zhiwu Boahu Xuebao 31:342–346
Zhou Y, Gu F, Zhou Ch, Yao H, Duan H, Wang F, Liu Y, Xing Y, Chu S (2010) Genetic diversity of R. glutinosa cultivars based on sequence-related amplified polymorphism markers. Sci Hort 125:789–794. doi:10.1016/j.scienta.2010.05.02
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by J. V. Jorrin-Novo.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
About this article
Cite this article
Piątczak, E., Grzegorczyk-Karolak, I. & Wysokińska, H. Micropropagation of Rehmannia glutinosa Libosch.: production of phenolics and flavonoids and evaluation of antioxidant activity. Acta Physiol Plant 36, 1693–1702 (2014). https://doi.org/10.1007/s11738-014-1544-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11738-014-1544-6