Abstract
Objective
To investigate the effect and the mechanism of electroacupuncture (EA) on corpus striatum white matter injury in rats with focal cerebral ischemia (FCI).
Methods
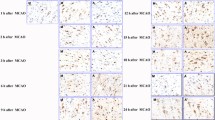
Forty-four specific-pathogen-free Sprague-Dawley rats were divided into a normal group (n=10), a sham-operation group (sham group, n=10), and a modeling group (n=24) using the random number table method. The normal group was a blank control. In the sham group, only the vessels and vagus nerve were isolated without embolization. The FCI rat model in the modeling group was replicated using the middle cerebral artery occlusion embolization method. The 20 successfully modeled rats were randomly divided into a model group and an EA group, with 10 rats in each group. Rats in the model group did not receive further treatment. Rats in the EA group received EA stimulation at Baihui (GV20) and the left Zusanli (ST36) 24 h after the successful modeling, 30 min each time, once a day for 14 d. On the 14th day of the experiment, rats in each group were scored for neurological deficits and then sacrificed, and brain tissues containing corpus striatum around the ischemic focus were paraffin-embedded from 5 rats in each group. Luxol fast blue (LFB) staining was used to detect damage changes in the white matter. The positive immunoreactive expression of myelin basic protein (MBP), myelin-associated growth inhibitor A (Nogo-A) and its receptor (NgR) in rat corpus striatum tissue was detected by immunohistochemistry staining, and then the protein expression of MBP, Nogo-A, and NgR in the corpus striatum tissue around the ischemic focus was determined by Western blotting.
Results
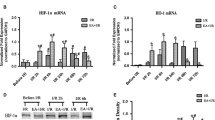
Compared with the normal group and the sham group, the model group had a significantly higher neurological deficit score (P<0.05) and fiber bundle injuries in the corpus striatum white matter, evidenced by a significantly lower mean optical density value of corpus striatum LFB staining (P<0.05), a significantly lower MBP expression level (P<0.05), and significantly higher Nogo-A and NgR protein expression levels (P<0.05). Compared with the model group, the neurological deficit score was significantly lower (P<0.05), the mean optical density value of LFB staining was significantly higher (P<0.05), the MBP expression level was increased (P<0.05), and the expression levels of Nogo-A and NgR proteins were decreased (P<0.05) in the EA group.
Conclusion
EA reduces the ischemia-induced corpus striatum white matter injury and improves neurological deficits. The mechanism may be related to the inhibition of Nogo-A/NgR activation.
摘要
目的
探讨电针(EA)对局灶性脑缺血大鼠纹状体白质损伤的影响及作用机制。
方法
无特定病原体Sprague-Dawley大鼠44只, 采用随机数字表法分为正常组(normal组, 10只)、假手术组(sham组, 10只)和造模组(24只)。normal组为空白对照; sham组仅分离血管和迷走神经而不栓塞; 造模组采用大脑中动脉栓塞法复制局灶性脑缺血大鼠模型。将模型制备成功的20只大鼠, 随机分为模型组(model组)和电针组(EA组), 每组10只。Model组大鼠不做进一步处理; EA组大鼠在造模成功后24 h 接受EA刺激百会和左侧足三里, 每次30 min, 每日1次, 连续14 d。实验第14天, 对各组大鼠进行神经功能缺损评分后处死大鼠, 每组5只取缺血灶周围含纹状体的脑组织石蜡包埋。采用神经髓鞘固蓝(LFB)染色检测白质损伤变化; 免疫组织化学染色法检测大鼠纹状体中髓鞘碱性蛋白(MBP)、髓鞘相关生长抑制因子(Nogo-A)及其受体(NgR)免疫反应阳性产物的表达。采用蛋白质免疫印迹试验检测缺血灶周围纹状体组织髓鞘相关蛋白MBP、Nogo-A和NgR的表达。
结果
与normal组和sham组比较, model组神经功能缺损评分明显增加(P<0.05), 呈现出纹状体白质纤维束损伤, 表现为纹状体LFB染色的平均光密度值明显降低(P<0.05), MBP表达水平明显降低(P<0.05), Nogo-A和NgR蛋白表达水平明显升高(P<0.05)。与model组相比, EA组神经功能缺损评分明显降低(P<0.05),LFB染色的平均光密度值显著升高(P<0.05), MBP表达水平升高(P<0.05), Nogo-A和NgR蛋白表达下降(P<0.05)。
结论
EA能减轻脑缺血诱发的纹状体白质损伤程度, 改善神经功能缺损; 其作用机制可能与抑制Nogo-A/NgR通路激活有关。
Similar content being viewed by others
References
HE X S, LI Y N, LU H Y, ZHANG Z J, WANG Y T, YANG G Y. Netrin-1 overexpression promotes white matter repairing and remodeling after focal cerebral ischemia in mice. J Cereb Blood Flow Metab, 2013, 33(12): 1921–1927.
LI Y N, TANG G H, LIU Y Q, HE X S, HUANG J, LIN X J, ZHANG Z J, YANG G Y, WANG Y T. Cxcl12 gene therapy ameliorates ischemia-induced white matter injury in mouse brain. Stem Cells Transl Med, 2015, 4(10): 1122–1130.
LIU S, JIN R, XIAO A Y, ZHONG W, LI G H. Inhibition of CD147 improves oligodendrogenesis and promotes white matter integrity and functional recovery in mice after ischemic stroke. Brain Behav Immun, 2019, 82: 13–24.
YOSHIOKA H, NIIZUMA K, KATSU M, SAKATA H, OKAMI N, CHAN P H. Consistent injury to medium spiny neurons and white matter in the mouse corpus striatum after prolonged transient global cerebral ischemia. J Neurotrauma, 2011, 28(4): 649–660.
ZHAO H, GAO X Y, LIU Z H, LIN J W, WANG S P, WANG D X, ZHANG Y B. Effects of the transcription factor Olig1 on the differentiation and remyelination of oligodendrocyte precursor cells after focal cerebral ischemia in rats. Mol Med Rep, 2019, 20(5): 4603–4611.
KUMAR P, MOON L D. Therapeutics targeting Nogo-A hold promise for stroke restoration. CNS Neurol Disord Drug Targets, 2013, 12(2): 200–208.
WANG T Z, WANG J, YIN C, LIU R, ZHANG J H, QIN X Y. Down-regulation of Nogo receptor promotes functional recovery by enhancing axonal connectivity after experimental stroke in rats. Brain Res, 2010, 1360: 147–158.
CHAVEZ L M, HUANG S S, MACDONALD I, LIN J G, LEE Y C, CHEN Y H. Mechanisms of acupuncture therapy in ischemic stroke rehabilitation: a literature review of basic studies. Int J Mol Sci, 2017, 18(11): 2270.
WANG H L, LIU F L, LI R Q, WAN M Y, LI J Y, SHI J, WU M L, CHEN J H, SUN W J, FENG H X, ZHAO W, HUANG J, LIU R C, HAO W X, FENG X D. Electroacupuncture improves learning and memory functions in a rat cerebral ischemia/reperfusion injury model through PI3K/Akt signaling pathway activation. Neural Regen Res, 2021, 16(6): 1011–1016.
DONG W Q, MIAO H C, WU F, LI H B. Effects of electroacupuncture on myelin-related protein in corpus callosum of rats with focal cerebral ischemia. Zhen Ci Yan Jiu, 2021, 46(9): 721–727.
LONGA E Z, WEINSTEIN P R, CARLSON S, CUMMINS R. Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke, 1989, 20(1): 84–91.
GUO Y. Experimental Acupuncture Science. Beijing: China Press of Traditional Chinese Medicine, 2016: 25–34.
TAMBASCO N, ROMOLIM, CALABRESI P. Selective basal ganglia vulnerability to energy deprivation: experimental and clinical evidences. Prog Neurobiol, 2018, 169: 55–75.
ZHANG J, CHEN S P, SHI W L, LI M Z, ZHAN Y, YANG L, ZOU H Y, LEI J F, CHAI X L, GAO K, LIU J J, WANG W, WANG Y, ZHAO H. Effects of Xiaoshuan enteric-coated capsule on white and gray matter injury evaluated by diffusion tensor imaging in ischemic stroke. Cell Transplant, 2019, 28(6): 671–683.
CAO J, HU Y P, SHAZEEB M S, PEDRAZA C E, PANDE N, WEINSTOCK D, POLITES G H, ZHANG W F, CHANDROSS K J, YING X Y. In vivo optical imaging of myelination events in a myelin basic protein promoter-driven luciferase transgenic mouse model. ASN Neuro, 2018, 10: 1759091418777329.
CHENG J Y, SHEN W M, JIN L Q, PAN J J, ZHOU Y, PAN G Y, XIE Q F, HU Q, WU S M, ZHANG H M, CHEN X. Treadmill exercise promotes neurogenesis and myelin repair via upregulating Wnt/β-catenin signaling pathways in the juvenile brain following focal cerebral ischemia/reperfusion. Int J Mol Med, 2020, 45(5): 1447–1463.
KIM K, SHIN M S, CHO H S, KIM Y P. Effects of endurance exercise on expressions of glial fibrillary acidic protein and myelin basic protein in developing rats with maternal infection-induced cerebral palsy. J Exerc Rehabil, 2014, 10(1): 9–14.
DAI Q X, GENG W J, ZHUANG X X, WANG H F, MO Y C, XIN H, CHEN J F, WANG J L. Electroacupuncture-induced neuroprotection against focal cerebral ischemia in the rat is mediated by adenosine A1 receptors. Neural Regen Res, 2017, 12(2): 228–234.
AHN S M, KIM Y R, KIM H N, SHIN Y I, SHIN H K, CHO B T. Electroacupuncture ameliorates memory impairments by enhancing oligodendrocyte regeneration in a mouse model of prolonged cerebral hypoperfusion. Sci Rep, 2016, 6: 28646.
XU H, ZHANG Y M, SUN H, CHEN S H, WANG F M. Effects of acupuncture at GV20 and ST36 on the expression of matrix metalloproteinase 2, aquaporin 4, and aquaporin 9 in rats subjected to cerebral ischemia/reperfusion injury. PLoS One, 2014, 9(5): e97488.
GOPALAKRISHNA R, MADES A, OH A, ZHU A, NGUYEN J, LIN C, KINDY M S, MACK W J. Cyclic-AMP induces Nogo-A receptor NgR1 internalization and inhibits Nogo-A-mediated collapse of growth cone. Biochem Biophys Res Commun, 2020, 523(3): 678–684.
NAGARAJ V, THEIS T, JOHAL A S, SETH A, GORE J, ARSHA N, PATEL M, HAO H B, KURIAN N, SCHACHNER M. Application of antibodies to neuronally expressed Nogo-A increases neuronal survival and neurite outgrowth. Int J Mol Sci, 2020, 21(15): 5417.
MARINM A, CARMICHAEL S T. Mechanisms of demyelination and remyelination in the young and aged brain following white matter stroke. Neurobiol Dis, 2019, 126: 5–12.
SOZMEN E G, ROSENZWEIG S, LLORENTE I L, DITULLIO D J, MACHNICKI M, VINTERS H V, HAVTON L A, GIGER R J, HINMAN J D, CARMICHAEL S T. Nogo receptor blockade overcomes remyelination failure after white matter stroke and stimulates functional recovery in aged mice. Proc Natl Acad Sci USA, 2016, 113(52): E8453–E8462.
PODRAZA K M, MEHTA Y, HUSAK V A, LIPPMANN E, O’BRIEN T E, KARTJE G L, TSAI S Y. Improved functional outcome after chronic stroke with delayed anti-Nogo-A therapy: a clinically relevant intention-to-treat analysis. J Cereb Blood Flow Metab, 2018, 38(8): 1327–1338.
HUANG S I, HUANG D X, ZHAO J P, CHEN L D. Electroacupuncture promotes axonal regeneration in rats with focal cerebral ischemia through the downregulation of Nogo-A/NgR/RhoA/Rock signaling. Exp Ther Med, 2017, 14(2): 905–912.
Acknowledgments
This work was supported by the Academic Leader Reserve Project of Wannan Medical College [皖南医学院学 术带头人后备人选人才项目 No.校政(2019) 81号].
Author information
Authors and Affiliations
Corresponding author
Additional information
Conflict of Interest
The authors declare that there is no potential conflict of interest in this article.
Statement of Human and Animal Rights
The treatment of animals conformed to the ethical criteria in this experiment.
Rights and permissions
About this article
Cite this article
Ma, T., Dong, W., Miao, H. et al. Electroacupuncture stimulation attenuates corpus striatum white matter injury in rats with cerebral ischemia by inhibition of Nogo-A/NgR pathway. J. Acupunct. Tuina. Sci. 21, 173–179 (2023). https://doi.org/10.1007/s11726-023-1373-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11726-023-1373-5
Keywords
- Acupuncture Therapy
- Electroacupuncture
- Brain Ischemia
- Corpus Striatum
- White Matter
- Nogo Proteins
- Nogo Receptors
- Rats