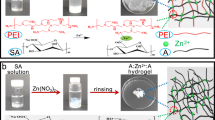
Abstract
A novel alginate/poly(acrylic acid/acrylamide) double-network hydrogel composite with silver nanoparticles was successfully fabricated using the sol-gel method. The presence of carboxyl and amide groups in the network structure provided abundant active sites for complexing silver ions, facilitating the in situ reduction and confinement of silver nanoparticles. In batch experiments, the optimal silver loading was 20%, and 5 mmol·L−1 of p-nitrophenol was completely degraded in 113 s with a rate constant value of 4.057 × 10−2 s−1. In the tap water system and simulated seawater system, the degradation time of p-nitrophenol at the same concentration was 261 and 276 s, respectively, with a conversion rate above 99%. In the fixed-bed experiment, the conversion rate remained above 74% after 3 h at a flowing rate of 7 mL·min−1. After 8 cycling tests, the conversion rate remained at 98.7%. Moreover, the catalyst exhibited outstanding performance in the degradation experiment of four typical organic dyes.

Similar content being viewed by others
References
Pandey S, Do J Y, Kim J, Kang M. Fast and highly efficient removal of dye from aqueous solution using natural locust bean gum based hydrogels as adsorbent. International Journal of Biological Macromolecules, 2020, 143: 60–75
Makhado E, Motshabi B R, Allouss D, Ramohlola K E, Modibane K D, Hato M J, Jugade R M, Shaik F, Pandey S. Development of a ghatti gum/poly (acrylic acid)/TiO2 hydrogel nanocomposite for malachite green adsorption from aqueous media: statistical optimization using response surface methodology. Chemosphere, 2022, 306: 135524
Pandey S, Son N, Kang M. Synergistic sorption performance of karaya gum crosslink poly(acrylamide-co-acrylonitrile)@metal nanoparticle for organic pollutants. International Journal of Biological Macromolecules, 2022, 210: 300–314
Pandey S, Mishra S B. Catalytic reduction of p-nitrophenol by using platinum nanoparticles stabilised by guar gum. Carbohydrate Polymers, 2014, 113: 525–531
Campos A, Troc N, Cottancin E, Pellarin M, Weissker H C, Lermé J, Kociak M, Hillenkamp M. Plasmonic quantum size effects in silver nanoparticles are dominated by interfaces and local environments. Nature Physics, 2019, 15(3): 275–280
Pandey S, Son N, Kim S, Balakrishnan D, Kang M. Locust bean gum-based hydrogels embedded magnetic iron oxide nanoparticles nanocomposite: advanced materials for environmental and energy applications. Environmental Research, 2022, 214(Part 3): 114000
Pourmadadi M, Eshaghi M M, Ostovar S, Shamsabadipour A, Safakhah S, Mousavi M S, Rahdar A, Pandey S. UiO-66 metal-organic framework nanoparticles as gifted MOFs to the biomedical application: a comprehensive review. Journal of Drug Delivery Science and Technology, 2022, 76: 103758
Kumar A, Sharipov M, Turaev A, Azizov S, Azizov I, Makhado E, Rahdar A, Kumar D, Pandey S. Polymer-based hybrid nanoarchitectures for cancer therapy applications. Polymers, 2022, 14(15): 3027
Sharma A, Nagraik R, Sharma S, Sharma G, Pandey S, Azizov S, Chauhan P K, Kumar D. Green synthesis of ZnO nanoparticles using ficus palmata: antioxidant, antibacterial and antidiabetic studies. Results in Chemistry, 2022, 4: 100509
Hassanisaadi M, Bonjar A H S, Rahdar A, Varma R S, Ajalli N, Pandey S. Eco-friendly biosynthesis of silver nanoparticles using aloysia citrodora leaf extract and evaluations of their bioactivities. Materials Today. Communications, 2022, 33: 104183
Moriai T, Tsukamoto T, Tanabe M, Kambe T, Yamamoto K. Selective hydroperoxygenation of olefins realized by a coinage multimetallic 1-nanometer catalyst. Angewandte Chemie International Edition, 2020, 59(51): 23051–23055
Jia W, Tian F, Zhang M, Li X, Ye S, Ma Y, Wang W, Zhang Y, Meng C, Zeng G, Liu J. Nitrogen-doped porous carbon-encapsulated copper composite for efficient reduction of 4-nitrophenol. Journal of Colloid and Interface Science, 2021, 594: 254–264
Hussain I, Shahid M, Ali F, Irfan A, Begum R, Farooqi Z H. Polymer hydrogels for stabilization of inorganic nanoparticles and their application in catalysis for degradation of toxic chemicals. Environmental Technology, 2021, 42(1): 1–11
Farooqi Z H, Sultana H, Begum R, Usman M, Ajmal M, Nisar J, Irfan A, Azam M. Catalytic degradation of malachite green using a crosslinked colloidal polymeric system loaded with silver nanoparticles. International Journal of Environmental Analytical Chemistry, 2022, 102(16): 4104–4120
Hussain I, Farooqi Z H, Ali F, Begum R, Irfan A, Wu W, Wang X, Shahid M, Nisar J. Poly(styrene@N-isopropylmethacrylamide-co-methacrylic acid)@Ag hybrid particles with excellent catalytic potential. Journal of Molecular Liquids, 2021, 335: 116106
Shah L A, Haleem A, Sayed M, Siddiq M. Synthesis of sensitive hybrid polymer microgels for catalytic reduction of organic pollutants. Journal of Environmental Chemical Engineering, 2016, 4(3): 3492–3497
Wang L, Chen S, Zhou J, Yang J, Chen X, Ji Y, Liu X, Zha L. Silver nanoparticles loaded thermoresponsive hybrid nanofibrous hydrogel as a recyclable dip-catalyst with temperature-tunable catalytic activity. Macromolecular Materials and Engineering, 2017, 302(10): 1700181
Khan M, Shah L A, Khan M A, Khattak N S, Zhao H. Synthesis of an un-modified gum arabic and acrylic acid based physically cross-linked hydrogels with high mechanical, self-sustainable and self-healable performance. Materials Science and Engineering C, 2020, 116: 111278
Zhang X, Zhang Y, Zhang W, Dai Y, Xia F. Gold nanoparticles-deranged double network for janus adhesive-tough hydrogel as strain sensor. Chemical Engineering Journal, 2021, 420(Part 3): 130447
Khan M, Shah L A, Rahman T U, Yoo H M, Ye D, Vacharasin J. Hydrophobically associated functionalized CNT-reinforced double-network hydrogels as advanced flexible strain sensors. ACS Applied Polymer Materials, 2022, 4(10): 7397–7407
Subhan H, Alam S, Shah L A, Khattak N S, Zekker I. Sodium alginate grafted hydrogel for adsorption of methylene green and use of the waste as an adsorbent for the separation of emulsified oil. Journal of Water Process Engineering, 2022, 46: 102546
Wu D, Yi M, Duan H, Xu J, Wang Q. Tough TiO2-rGO-PDMAA nanocomposite hydrogel via one-pot UV polymerization and reduction for photodegradation of methylene blue. Carbon, 2016, 108: 394–403
He X, Gopinath K, Sathishkumar G, Guo L, Zhang K, Lu Z, Li C, Kang E T, Xu L. UV-assisted deposition of antibacterial Ag-tannic acid nanocomposite coating. ACS Applied Materials & Interfaces, 2021, 13(17): 20708–20717
Gao C, An Q, Xiao Z, Zhai S, Zhai B, Shi Z. Alginate and polyethyleneimine dually mediated synthesis of nanosilver-containing composites for efficient p-nitrophenol reduction. Carbohydrate Polymers, 2018, 181: 744–751
Makhado E, Pandey S, Modibane K D, Kang M, Hato M J. Sequestration of methylene blue dye using sodium alginate poly(acrylic acid)@ZnO hydrogel nanocomposite: kinetic, isotherm, and thermodynamic investigations. International Journal of Biological Macromolecules, 2020, 162: 60–73
Wang Z, Peng X, Guo S, Sun M, Cheng J, Zou L, Chi B, Pu J. Ultraviolet light-assisted Ag@La0.6Sr0.4Fe0.9Mn0.1O3 nanohybrids: a facile and versatile method for preparation of highly stable catalysts in Li-O2 batteries. ACS Applied Energy Materials, 2021, 4(9): 9376–9383
Zhang Z, Hu J, Wang Y, Shi R, Ma Y, Huang H, Wang H, Wei J, Yu Q. Relationship between microstructure of AgCl film and electrochemical behavior of Ag|AgCl electrode for chloride detection. Corrosion Science, 2021s, 184: 109393
Nguyen T T N, Lee M S. Purification of the sodium hydroxide leaching solution of black dross by removal of silicate(IV) with polyacrylamide (PAM). Mineral Processing and Extractive Metallurgy Review, 2021, 42(1): 9–16
Ryu J H, Han N K, Lee J S, Jeong Y G. Microstructure, thermal and mechanical properties of composite films based on carboxymethylated nanocellulose and polyacrylamide. Carbohydrate Polymers, 2019, 211: 84–90
Xu S, Liang W, Xu G, Huang C, Zhang J, Lang M. A fast and dual crosslinking hydrogel based on vinyl ether sodium alginate. Applied Surface Science, 2020, 515: 145811
Das R, Sypu V S, Paumo H K, Bhaumik M, Maharaj V, Maity A. Silver decorated magnetic nanocomposite (Fe3O4@PPy-MAA/Ag) as highly active catalyst towards reduction of 4-nitrophenol and toxic organic dyes. Applied Catalysis B: Environmental, 2019, 244: 546–558
Dong G, Cao Y, Zheng S, Zhou J, Li W, Zaera F, Zhou X. Catalyst consisting of Ag nanoparticles anchored on amine-derivatized mesoporous silica nanospheres for the selective hydrogenation of dimethyl oxalate to methyl glycolate. Journal of Catalysis, 2020, 391: 155–162
e T, Ma D, Yang S, Hao X O, Ma D, Yang S, Hao X. Graphene oxide-montmorillonite/sodium alginate aerogel beads for selective adsorption of methylene blue in wastewater. Journal of Alloys and Compounds, 2020, 832: 154833
Gao C, Xiao L, Zhou J, Wang H, Zhai S, An Q. Immobilization of nanosilver onto glycine modified lignin hydrogel composites for highly efficient p-nitrophenol hydrogenation. Chemical Engineering Journal, 2021, 403: 126370
Shah L A. Developing Ag-tercopolymer microgels for the catalytic reduction of p-nitrophenol and Eosin Y throughout the entire pH range. Journal of Molecular Liquids, 2019, 288: 111045
Yan Q, Wang X Y, Feng J J, Mei L P, Wang A J. Simple fabrication of bimetallic platinum-rhodium alloyed nano-multipods: a highly effective and recyclable catalyst for reduction of 4-nitrophenol and rhodamine B. Journal of Colloid and Interface Science, 2021, 582(Part B): 701–710
Khalil A, Ali N, Asiri A M, Kamal T, Khan S B, Ali J. Synthesis and catalytic evaluation of silver@nickel oxide and alginate biopolymer nanocomposite hydrogel beads. Cellulose, 2021, 28(18): 11299–11313
Khan Z, Bashir O, Khan M N, Khan T A, Al-Thabaiti S A. Cationic surfactant assisted morphology of Ag@Cu, and their catalytic reductive degradation of rhodamine B. Journal of Molecular Liquids, 2017, 248: 1096–1108
Subhan F, Aslam S, Yan Z, Yaseen M, Zada A, Ikram M. Fabrication of highly dispersed Pt NPs in nanoconfined spaces of as-made KIT-6 for nitrophenol and MB catalytic reduction in water. Separation and Purification Technology, 2021, 265: 118532
Yu Y, Liu S, Pei Y, Luo X. Growing Pd NPs on cellulose microspheres via in-situ reduction for catalytic decolorization of methylene blue. International Journal of Biological Macromolecules, 2021, 166: 1419–1428
Lajevardi A, Tavakkoli Yaraki M, Masjedi A, Nouri A, Hossaini Sadr M. Green synthesis of MOF@Ag nanocomposites for catalytic reduction of methylene blue. Journal of Molecular Liquids, 2019, 276: 371–378
Akilandaeaswari B, Muthu K. One-pot green synthesis of Au-Ag bimetallic nanoparticles from lawsonia inermis seed extract and its catalytic reduction of environmental polluted methyl orange and 4-nitrophenol. Journal of the Taiwan Institute of Chemical Engineers, 2021, 127: 292–301
Khan S B, Khan M S J, Kamal T, Asiri A M, Bakhsh E M. Polymer supported metallic nanoparticles as a solid catalyst for the removal of organic pollutants. Cellulose, 2020, 27(10): 5907–5921
Malik A, Nath M. Synthesis of Ag/ZIF-7 by immobilization of Ag nanoparticles onto ZIF-7 microcrystals: a heterogeneous catalyst for the reduction of nitroaromatic compounds and organic dyes. Journal of Environmental Chemical Engineering, 2020, 8(6): 104547
Malik M A, Alshehri A A, Patel R. Facile one-pot green synthesis of Ag-Fe bimetallic nanoparticles and their catalytic capability for 4-nitrophenol reduction. Journal of Materials Research and Technology, 2021, 12: 455–470
Li X, Zeng C, Jiang J, Ai L. Magnetic cobalt nanoparticles embedded in hierarchically porous nitrogen-doped carbon frameworks for highly efficient and well-recyclable catalysis. Journal of Materials Chemistry A: Materials for Energy and Sustainability, 2016, 4(19): 7476–7482
Rezaei F, Dinari M. Cu nanoparticles embedded in the porous organic polymer as highly effective catalysts for nitroaromatics reduction. Microporous and Mesoporous Materials, 2021, 325: 111339
Xu Y, Shi X, Hua R, Zhang R, Yao Y, Zhao B, Liu T, Zheng J, Lu G. Remarkably catalytic activity in reduction of 4-nitrophenol and methylene blue by Fe3O4@COF supported noble metal nanoparticles. Applied Catalysis B: Environmental, 2020, 260: 118142
Yu H, Oh S, Han Y, Lee S, Jeong H S, Hong H J. Modified cellulose nanofibril aerogel: tunable catalyst support for treatment of 4-nitrophenol from wastewater. Chemosphere, 2021, 285: 131448
Peng C, Kuai Z, Li X, Lian S, Jiang D, Tang J, Li L, Wu R, Wu A, Chen S. Facile synthesis of Ag nanoparticles/Ti3C2Tx/polyacry-lamide composite hydrogel as efficient catalyst for methylene blue and 4-nitrophenol reduction. Materials & Design, 2021, 210: 110061
Acknowledgements
This research was financially supported by the National Natural Science Foundation of China (Grant Nos. 21776026, 22075034, and 22178037), and Liaoning Revitalization Talents Program (Grant Nos. XLYC1902037 and XLYC2002114), and Natural Science Foundation of Liaoning Province of China (Grant No. 2021-MS-303).
Author information
Authors and Affiliations
Corresponding authors
Electronic Supplementary Material
11705_2022_2290_MOESM1_ESM.pdf
Nanosilver anchored alginate/poly(acrylic acid/acrylamide) double-network hydrogel composites for efficient catalytic degradation of organic dyes
Rights and permissions
About this article
Cite this article
Zhang, F., Gao, C., Zhai, SR. et al. Nanosilver anchored alginate/poly(acrylic acid/acrylamide) double-network hydrogel composites for efficient catalytic degradation of organic dyes. Front. Chem. Sci. Eng. 17, 893–905 (2023). https://doi.org/10.1007/s11705-022-2290-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11705-022-2290-8