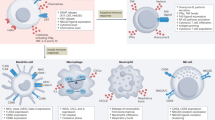
Abstract
Compared to conventional hyperthermia that is limited by low selectivity and severe side effects, nano-enabled hyperthermia yields great potentials to tackle these limitations for cancer treatment. Another major advance is the observation of immunological responses associated with nano-enabled hyperthermia, which introduces a new avenue, allowing a potential paradigm shift from the acutely effective and cytotoxicity-centric response to the next-phase discovery, i.e., long-lasting and/or systemic anti-tumor immunity. This perspective first discusses the temperature-gradient and the spatially-structured immunological landscape in solid tumors receiving nano-enabled hyperthermia. This includes the discussion about underlying mechanism such as immunogenic cell death, which initiates a profound immunological chain reaction. In order to propagate the immune activation as a viable therapeutic principle, we further discussed the tumor type-specific complexity in the immunological tumor microenvironment, including the creative design of nano-enabled combination therapy to synergize with nano-enabled hyperthermia.

Similar content being viewed by others
References
Chu K F, Dupuy D E. Thermal ablation of tumours: biological mechanisms and advances in therapy. Nature Reviews. Cancer, 2014, 14(3): 199–208
Falk M H, Issels R D. Hyperthermia in oncology. International Journal of Hyperthermia, 2001, 17(1): 1–18
Field S B, Bleehen N M. Hyperthermia in the treatment of cancer. Cancer Treatment Reviews, 1979, 6(2): 63–94
Wust P, Hildebrandt B, Sreenivasa G, Rau B, Gellermann J, Riess H, Felix R, Schlag P M. Hyperthermia in combined treatment of cancer. Lancet. Oncology, 2002, 3(8): 487–497
Cherukuri P, Glazer E S, Curley S A. Targeted hyperthermia using metal nanoparticles. Advanced Drug Delivery Reviews, 2010, 62(3): 339–345
Thiesen B, Jordan A. Clinical applications of magnetic nanoparticles for hyperthermia. International Journal of Hyperthermia, 2008, 24(6): 467–474
Beik J, Abed Z, Ghoreishi F S, Hosseini-Nami S, Mehrzadi S, Shakeri-Zadeh A, Kamrava S K. Nanotechnology in hyperthermia cancer therapy: from fundamental principles to advanced applications. Journal of Controlled Release, 2016, 235: 205–221
Huang X, Jain P K, El-Sayed I H, El-Sayed M A. Plasmonic photothermal therapy (PPTT) using gold nanoparticles. Lasers in Medical Science, 2008, 23(3): 217–228
Jaque D, Maestro L M, Del Rosal B, Haro Gonzalez P, Benayas A, Plaza J, Rodriguez E M, Sole J. Nanoparticles for photothermal therapies. Nanoscale, 2014, 6(16): 9494–9530
Liu X, Huang N, Li H, Wang H, Jin Q, Ji J. Multidentate polyethylene glycol modified gold nanorods for in vivo near-infrared photothermal cancer therapy. ACS Applied Materials & Interfaces, 2014, 6(8): 5657–5668
Kim H C, Kim E, Jeong S W, Ha T L, Park S I, Lee S G, Lee S J, Lee S W. Magnetic nanoparticle-conjugated polymeric micelles for combined hyperthermia and chemotherapy. Nanoscale, 2015, 7(39): 16470–16480
Tamarov K P, Osminkina L A, Zinovyev S V, Maximova K A, Kargina J V, Gongalsky M B, Ryabchikov Y, Al Kattan A, Sviridov A P, Sentis M. Radio frequency radiation-induced hyperthermia using Si nanoparticle-based sensitizers for mild cancer therapy. Scientific Reports, 2014, 4(1): 1–7
Kumar R, Chauhan A, Jha S K, Kuanr B K. Localized cancer treatment by radio-frequency hyperthermia using magnetic nanoparticles immobilized on graphene oxide: from novel synthesis to in vitro studies. Journal of Materials Chemistry. B, Materials for Biology and Medicine, 2018, 6(33): 5385–5399
Cardinal J, Klune J R, Chory E, Jeyabalan G, Kanzius J S, Nalesnik M, Geller D A. Noninvasive radiofrequency ablation of cancer targeted by gold nanoparticles. Surgery, 2008, 144(2): 125–132
Kaczmarek K, Hornowski T, Kubovcikova M, Timko M, Koralewski M, Jozefczak A. Heating induced by therapeutic ultrasound in the presence of magnetic nanoparticles. ACS Applied Materials & Interfaces, 2018, 10(14): 11554–11564
Beik J, Abed Z, Shakeri-Zadeh A, Nourbakhsh M, Shiran M B. Evaluation of the sonosensitizing properties of nano-graphene oxide in comparison with iron oxide and gold nanoparticles. Physica E, Low-Dimensional Systems and Nanostructures, 2016, 81: 308–314
Devarakonda S B, Myers M R, Lanier M, Dumoulin C, Banerjee R K. Assessment of gold nanoparticle-mediated-enhanced hyperthermia using MR-guided high-intensity focused ultrasound ablation procedure. Nano Letters, 2017, 17(4): 2532–2538
Hildebrandt B, Wust P, Ahlers O, Dieing A, Sreenivasa G, Kerner T, Felix R, Riess H. The cellular and molecular basis of hyperthermia. Critical Reviews in Oncology/Hematology, 2002, 43(1): 33–56
Lepock J R. Cellular effects of hyperthermia: relevance to the minimum dose for thermal damage. International Journal of Hyperthermia, 2003, 19(3): 252–266
Roti J L. Cellular responses to hyperthermia (40 °C–46 °C): cell killing and molecular events. International Journal of Hyperthermia, 2008, 24(1): 3–15
Fairchild K D, Viscardi R M, Hester L, Singh I S, Hasday J D. Effects of hypothermia and hyperthermia on cytokine production by cultured human mononuclear phagocytes from adults and newborns. Journal of Interferon & Cytokine Research, 2000, 20(12): 1049–1055
Ito A, Honda H, Kobayashi T. Cancer immunotherapy based on intracellular hyperthermia using magnetite nanoparticles: a novel concept of “heat-controlled necrosis” with heat shock protein expression. Cancer Immunology, Immunotherapy, 2006, 55(3): 320–328
Ito A, Shinkai M, Honda H, Yoshikawa K, Saga S, Wakabayashi T, Yoshida J, Kobayashi T. Heat shock protein 70 expression induces antitumor immunity during intracellular hyperthermia using magnetite nanoparticles. Cancer Immunology, Immunotherapy, 2003, 52(2): 80–88
Galluzzi L, Buqué A, Kepp O, Zitvogel L, Kroemer G. Immunogenic cell death in cancer and infectious disease. Nature Reviews. Immunology, 2017, 17(2): 97–111
Todryk S, Melcher A, Dalgleish A, Vile R G. Heat shock proteins refine the danger theory. Immunology, 2000, 99(3): 334–337
Zhang Z, Wang J, Chen C. Gold nanorods based platforms for light-mediated theranostics. Theranostics, 2013, 3(3): 223–238
Dickerson E B, Dreaden E C, Huang X, El Sayed I H, Chu H, Pushpanketh S, McDonald J F, El Sayed M A. Gold nanorod assisted near-infrared plasmonic photothermal therapy (PPTT) of squamous cell carcinoma in mice. Cancer Letters, 2008, 269(1): 57–66
Yang K, Feng L, Shi X, Liu Z. Nano-graphene in biomedicine: theranostic applications. Chemical Society Reviews, 2013, 42(2): 530–547
Guo L, Yan D D, Yang D, Li Y, Wang X, Zalewski O, Yan B, Lu W. Combinatorial photothermal and immuno cancer therapy using chitosan-coated hollow copper sulfide nanoparticles. ACS Nano, 2014, 8(6): 5670–5681
Wang C, Xu L, Liang C, Xiang J, Peng R, Liu Z. Immunological responses triggered by photothermal therapy with carbon nanotubes in combination with anti-CTLA-4 therapy to inhibit cancer metastasis. Advanced Materials, 2014, 26(48): 8154–8162
Tao Y, Ju E, Ren J, Qu X. Immunostimulatory oligonucleotides-loaded cationic graphene oxide with photothermally enhanced immunogenicity for photothermal/immune cancer therapy. Biomaterials, 2014, 35(37): 9963–9971
Chen Q, Xu L, Liang C, Wang C, Peng R, Liu Z. Photothermal therapy with immune-adjuvant nanoparticles together with checkpoint blockade for effective cancer immunotherapy. Nature Communications, 2016, 7(1): 1–13
Liu Y, Maccarini P, Palmer G M, Etienne W, Zhao Y, Lee C T, Ma X, Inman B A, Vo-Dinh T. Synergistic immuno photothermal nanotherapy (SYMPHONY) for the treatment of unresectable and metastatic cancers. Scientific Reports, 2017, 7(1): 8606
Nam J, Son S, Ochyl L J, Kuai R, Schwendeman A, Moon J J. Chemo-photothermal therapy combination elicits anti-tumor immunity against advanced metastatic cancer. Nature Communications, 2018, 9(1): 1074
Yu G T, Rao L, Wu H, Yang L L, Bu L L, Deng W W, Wu L, Nan X, Zhang W F, Zhao X Z, Liu W, Sun Z J. Myeloid-derived suppressor cell membrane-coated magnetic nanoparticles for cancer theranostics by Inducing macrophage polarization and synergizing immunogenic cell death. Advanced Functional Materials, 2018, 28(37): 1801389
Dong X, Liang J, Yang A, Qian Z, Kong D, Lv F. Fluorescence imaging guided CpG nanoparticles-loaded IR820-hydrogel for synergistic photothermal immunotherapy. Biomaterials, 2019, 209: 111–125
Zhang D, Wu T, Qin X, Qiao Q, Shang L, Song Q, Yang C, Zhang Z. Intracellularly generated immunological gold nanoparticles for combinatorial photothermal therapy and immunotherapy against tumor. Nano Letters, 2019, 19(9): 6635–6646
Chao Y, Chen G, Liang C, Xu J, Dong Z, Han X, Wang C, Liu Z. Iron nanoparticles for low-power local magnetic hyperthermia in combination with immune checkpoint blockade for systemic antitumor therapy. Nano Letters, 2019, 19(7): 4287–4296
Ong C, Cha B G, Kim J. Mesoporous silica nanoparticles doped with gold nanoparticles for combined cancer immunotherapy and photothermal therapy. ACS Applied Bio Materials, 2019, 2(8): 3630–3638
Ma Y, Zhang Y, Li X, Zhao Y, Li M, Jiang W, Tang X, Dou J, Lu L, Wang F, Wang Y. Near-infrared II phototherapy induces deep tissue immunogenic cell death and potentiates cancer immunotherapy. ACS Nano, 2019, 13(10): 11967–11980
Wang Z, Guo B, Middha E, Huang Z, Hu Q, Fu Z, Liu B. Microfluidics-prepared uniform conjugated polymer nanoparticles for photo-triggered immune microenvironment modulation and cancer therapy. ACS Applied Materials & Interfaces, 2019, 11(12): 11167–11176
Li Y, Liu X, Pan W, Li N, Tang B. Photothermal therapy-induced immunogenic cell death based on natural melanin nanoparticles against breast cancer. Chemical Communications, 2020, 56(9): 1389–1392
Bezu L, Gomes-da-Silva L C, Dewitte H, Breckpot K, Fucikova J, Spisek R, Galluzzi L, Kepp O, Kroemer G. Combinatorial strategies for the induction of immunogenic cell death. Frontiers in Immunology, 2015, 6: 187
Duan X, Chan C, Lin W. Nanoparticle-mediated immunogenic cell death enables and potentiates cancer immunotherapy. Angewandte Chemie International Edition, 2019, 58(3): 670–680
Rodallec A, Sicard G, Fanciullino R, Benzekry S, Lacarelle B, Milano G, Ciccolini J. Turning cold tumors into hot tumors: harnessing the potential of tumor immunity using nanoparticles. Expert Opinion on Drug Metabolism & Toxicology, 2018, 14(11): 1139–1147
Wang S, Riedinger A, Li H, Fu C, Liu H, Li L, Liu T, Tan L, Barthel M J, Pugliese G, De Donato F, Scotto D’Abbusco M, Meng X, Manna L, Meng H, Pellegrino T. Plasmonic copper sulfide nanocrystals exhibiting near-infrared photothermal and photodynamic therapeutic effects. ACS Nano, 2015, 9(2): 1788–1800
Kroemer G, Galluzzi L, Kepp O, Zitvogel L. Immunogenic cell death in cancer therapy. Annual Review of Immunology, 2013, 31(1): 51–72
Garg A D, Dudek-Peric A M, Romano E, Agostinis P. Immunogenic cell death. International Journal of Developmental Biology, 2015, 59(1–3): 131–140
Liu X, Jiang J, Liao Y P, Tang I, Zheng E, Qiu W, Lin M, Wang X, Ji Y, Mei K C, et al. Combination chemo-immunotherapy for pancreatic cancer using the immunogenic effects of an irinotecan silicasome nanocarrier plus anti-PD-1. Advancement of Science, 2021, 8(6): 2002147
Wong D Y, Ong W W, Ang W H. Induction of immunogenic cell death by chemotherapeutic platinum complexes. Angewandte Chemie International Edition in English, 2015, 54(22): 6483–6487
Verfaillie T, Rubio N, Garg A D, Bultynck G, Rizzuto R, Decuypere J P, Piette J, Linehan C, Gupta S, Samali A, Agostinis P. PERK is required at the ER-mitochondrial contact sites to convey apoptosis after ROS-based ER stress. Cell Death and Differentiation, 2012, 19(11): 1880–1891
Garg A D, Krysko D V, Verfaillie T, Kaczmarek A, Ferreira G B, Marysael T, Rubio N, Firczuk M, Mathieu C, Roebroek A J, Annaert W, Golab J, de Witte P, Vandenabeele P, Agostinis P. A novel pathway combining calreticulin exposure and ATP secretion in immunogenic cancer cell death. EMBO Journal, 2012, 31(5): 1062–1079
Radogna F, Diederich M. Stress-induced cellular responses in immunogenic cell death: implications for cancer immunotherapy. Biochemical Pharmacology, 2018, 153: 12–23
Krysko D V, Garg A D, Kaczmarek A, Krysko O, Agostinis P, Vandenabeele P. Immunogenic cell death and DAMPs in cancer therapy. Nature Reviews. Cancer, 2012, 12(12): 860–875
Li W, Yang J, Luo L, Jiang M, Qin B, Yin H, Zhu C, Yuan X, Zhang J, Luo Z, Du Y, Li Q, Lou Y, Qiu Y, You J. Targeting photodynamic and photothermal therapy to the endoplasmic reticulum enhances immunogenic cancer cell death. Nature Communications, 2019, 10(1): 3349
Dimou A, Syrigos K N, Saif M W. Overcoming the stromal barrier: technologies to optimize drug delivery in pancreatic cancer. Therapeutic Advances in Medical Oncology, 2012, 4(5): 271–279
Meng H, Zhao Y, Dong J, Xue M, Lin Y S, Ji Z, Mai W X, Zhang H, Chang C H, Brinker C J, Zink J I, Nel A E. Two-wave nanotherapy to target the stroma and optimize gemcitabine delivery to a human pancreatic cancer model in mice. ACS Nano, 2013, 7(11): 10048–10065
Miao L, Lin C M, Huang L. Stromal barriers and strategies for the delivery of nanomedicine to desmoplastic tumors. Journal of Controlled Release, 2015, 219: 192–204
Meng H, Nel A E. Use of nano engineered approaches to overcome the stromal barrier in pancreatic cancer. Advanced Drug Delivery Reviews, 2018, 130: 50–57
Rosenberg A, Mahalingam D. Immunotherapy in pancreatic adenocarcinoma-overcoming barriers to response. Journal of Gastrointestinal Oncology, 2018, 9(1): 143–159
Torphy R J, Zhu Y, Schulick R D. Immunotherapy for pancreatic cancer: barriers and breakthroughs. Annals of Gastroenterological Surgery, 2018, 2(4): 274–281
Issels R D. Hyperthermia adds to chemotherapy. European Journal of Cancer, 2008, 44(17): 2546–2554
Nam J, Son S, Park K S, Zou W P, Shea L D, Moon J J. Cancer nanomedicine for combination cancer immunotherapy. Nature Reviews. Materials, 2019, 4(6): 398–414
Peng J, Xiao Y, Li W, Yang Q, Tan L, Jia Y, Qu Y, Qian Z. Photosensitizer micelles together with IDO inhibitor enhance cancer photothermal therapy and immunotherapy. Advancement of Science, 2018, 5(5): 1700891
Li Y, Li X, Zhou F, Doughty A, Hoover A R, Nordquist R E, Chen W R. Nanotechnology-based photoimmunological therapies for cancer. Cancer Letters, 2019, 442: 429–438
Liu X, Zhang Y, Wang Y, Zhu W, Li G, Ma X, Zhang Y, Chen S, Tiwari S, Shi K, Zhang S, Fan H M, Zhao Y X, Liang X J. Comprehensive understanding of magnetic hyperthermia for improving antitumor therapeutic efficacy. Theranostics, 2020, 10(8): 3793–3815
Mei K C, Liao Y P, Jiang J, Chiang M, Khazaieli M, Liu X, Wang X, Liu Q, Chang C H, Zhang X, Li J, Ji Y, Melano B, Telesca D, Xia T, Meng H, Nel A E. Liposomal delivery of mitoxantrone and a cholesteryl indoximod prodrug provides effective chemo-immunotherapy in multiple solid tumors. ACS Nano, 2020, 14(10): 13343–13366
Stephen Z R, Zhang M. Recent progress in the synergistic combination of nanoparticle-mediated hyperthermia and immunotherapy for treatment of cancer. Advanced Healthcare Materials, 2020, 10(2): 2001415
Wang S, Sun Z, Hou Y. Engineering nanoparticles toward the modulation of emerging cancer immunotherapy. Advanced Healthcare Materials, 2020, 10(5): e2000845
Mortezaee K, Narmani A, Salehi M, Bagheri H, Farhood B, Haghi-Aminjan H, Najafi M. Synergic effects of nanoparticles-mediated hyperthermia in radiotherapy/chemotherapy of cancer. Life Sciences, 2021, 269: 119020
Liu X S, Jiang J H, Chang C H, Liao Y P, Jared J L, Tang I, Zheng E, Qiu W, Lin M, Wang X, et al. Development of facile and versatile platinum drug delivering silicasome nanocarriers for efficient pancreatic cancer chemo-immunotherapy. Small, 2021, 17(14): 2005993
Chen J, Lin L, Yan N, Hu Y, Fang H, Guo Z, Sun P, Tian H, Chen X. Macrophages loaded CpG and GNR-PEI for combination of tumor photothermal therapy and immunotherapy. Science China Materials, 2018, 61(11): 1484–1494
Musetti S, Huang L. Nanoparticle-mediated remodeling of the tumor microenvironment to enhance immunotherapy. ACS Nano, 2018, 12(12): 11740–11755
Allen S D, Liu X, Jiang J, Liao Y P, Chang C H, Nel A E, Meng H. Immune checkpoint inhibition in syngeneic mouse cancer models by a silicasome nanocarrier delivering a GSK3 inhibitor. Biomaterials, 2021, 269: 120635
Chen P M, Pan W Y, Wu C Y, Yeh C Y, Korupalli C, Luo P K, Chou C J, Chia W T, Sung H W. Modulation of tumor microenvironment using a TLR-7/8 agonist-loaded nanoparticle system that exerts low-temperature hyperthermia and immunotherapy for in situ cancer vaccination. Biomaterials, 2020, 230: 119629
Shobaki N, Sato Y, Suzuki Y, Okabe N, Harashima H. Manipulating the function of tumor-associated macrophages by siRNA-loaded lipid nanoparticles for cancer immunotherapy. Journal of Controlled Release, 2020, 325: 235–248
Teo P Y, Yang C, Whilding L M, Parente-Pereira A C, Maher J, George A J, Hedrick J L, Yang Y Y, Ghaem-Maghami S. Ovarian cancer immunotherapy using PD-L1 siRNA targeted delivery from folic acid-functionalized polyethylenimine: strategies to enhance T cell killing. Advanced Healthcare Materials, 2015, 4(8): 1180–1189
Golden E B, Apetoh L. Radiotherapy and immunogenic cell death. Seminars in Radiation Oncology, 2015, 25(1): 11–17
Galluzzi L, Kepp O, Kroemer G. Immunogenic cell death in radiation therapy. OncoImmunology, 2013, 2(10): e26536
Acknowledgements
This work was supported by the National Natural Science Foundation of China (Grant Nos. 31671017 and 81872809) and the startup funding support from The Cancer Hospital of the University of Chinese Academy of Sciences (CAS), Institute of Basic Medicine and Cancer (IBMC), CAS. HM thanks the start-up packages of NCNST, CAS.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Liu, X., Sun, H., Wang, X. et al. Immunological effects of nano-enabled hyperthermia for solid tumors: opportunity and challenge. Front. Chem. Sci. Eng. 16, 333–344 (2022). https://doi.org/10.1007/s11705-021-2059-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11705-021-2059-5