Abstract
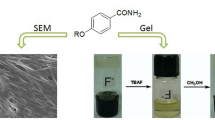
Four 2,5-dialkoxylphenyl-1,3,4-oxadiazoles are shown to be efficient organogelators. These compounds readily form stable gels in many organic solvents and their gelation property as well as supramolecular structures were investigated by scanning electron microscopy (SEM), X-ray diffraction (XRD), 1H nuclear magnetic resonance (1H NMR), and ultraviolet-visible spectroscopy (UV-vis). The results indicate that the gelator molecules self-assemble into gels with elongated fibrous networks and layer structures, and van der Waals interaction is the main driving force.
Similar content being viewed by others
References
Terech P, Weiss R G. Low molecular mass gelators of organic liquids and the properties of their gels. Chemical Reviews, 1997, 97(8): 3133–3160
George M, Weiss R G. Molecular organogels. Soft matter comprised of low-molecular-mass organic gelators and organic liquids. Accounts of Chemical Research, 2006, 39(8): 489–497
Wang X, Peng Q, Li Y. Interface-mediated growth of monodispersed nanostructures. Accounts of Chemical Research, 2007, 40(8): 635–643
Dastidar P. Supramolecular gelling agents: can they be designed? Chemical Society Reviews, 2008, 37(12): 2699–2715
Yang Z, Xu K, Wang L, Gu H, Wei H, Zhang M, Xu B. Selfassembly of small molecules affords multifunctional supramolecular hydrogels for topically treating simulated uranium wounds. Chemical Communications, 2005, 35: 4414–4416
Bonacucina G, Cespi M, Misici-Falzi M, Palmieri G F. Colloidal soft matter as drug delivery system. Journal of Pharmaceutical Sciences, 2009, 98(1): 1–42
Love C S, Chechik V, Smith D K, Wilson K, Ashworth I, Brennan C. Synthesis of gold nanoparticles within a supramolecular gelphase network. Chemical Communications, 2005, 15: 1971–1973
Vemula P K, John G. Smart amphiphiles: hydro/organogelators for in situ reduction of gold. Chemical Communications, 2006, 21: 2218–2220
Yang X, Lu R, Xue P, Li B, Xu D, Xu T, Zhao Y. Carbazole-based organogel as a scaffold to construct energy transfer arrays with controllable fluorescence emission. Langmuir, 2008, 24(23): 13730–13735
Danjo H, Hirata K, Yoshigai S, Azumaya I, Yamaguchi K. Back to back twin bowls of D3-symmetric tris(spiroborate)s for supramolecular chain structures. Journal of the American Chemical Society, 2009, 131(5): 1638–1639
Schenning A P H J, Jonkheijm P, Peeters E, Meijer E W. Hierarchical order in supramolecular assemblies of hydrogenbonded oligo(p-phenylene vinylene)s. Journal of the American Chemical Society, 2001, 123(3): 409–416
Jonkheijm P, Miura A, Zdanowska M, Hoeben F J M, De Feyter S, Schenning A P H J, De Schryver F C, Meijer E W. π-conjugated oligo-(p-phenylenevinylene) rosettes and their tubular self-assembly. Angewandte Chemie International Edition, 2004, 43(1): 74–78
Hirst A R, Coates I A, Boucheteau T R, Miravet J F, Escuder B, Castelletto V, Hamley I W, Smith D K. Low-molecular-weight gelators: elucidating the principles of gelation based on gelator solubility and a cooperative self-assembly model. Journal of the American Chemical Society, 2008, 130(28): 9113–9121
Ravindra K C, Vagdevi H M, Padmashali B. Synthesis, antimicrobial and antiinflammatory activities of 1,3,4-oxadiazoles linked to naphtho[2,1-b]furan. Indian Journal of Chemistry Sect. B, 2006, 45B(11): 2506–2511
Chen H, Li Z, Han Y. Synthesis and fungicidal activity against Rhizoctonia solani of 2-alkyl (Alkylthio)-5-pyrazolyl-1,3,4-oxadiazoles (Thiadiazoles). Journal of Agricultural and Food Chemistry, 2000, 48(11): 5312–5315
Xu Z W, Li Y, Ma X M, Gao X D, Tian H. Synthesis and properties of iridium complexes based 1,3,4-oxadiazoles derivatives. Tetrahedron, 2008, 64(8): 1860–1867
Mitschke U, Bauerle P. The electroluminescence of organic materials. Journal of Materials Chemistry, 2000, 10(7): 1471–1507
Lu W, Law Y C, Han J, Chui S S Y, Ma D L, Zhu N, Che C M. A dicationic organoplatinum(II) complex containing a bridging 2,5-bis-(4-ethynylphenyl)-[1,3,4]oxadiazole ligand behaves as a phosphorescent gelator for organic solvents. Asian Journal of Chemistry, 2008, 3(1): 59–69
Han J, Chui S S Y, Che C M. Thermotropic liquid crystals based on extended 2,5-disubstituted-1,3,4-oxadiazoles: structure-property relationships, variable-temperature powder X-ray diffraction, and small-angle X-ray scattering studies. Asian Journal of Chemistry, 2006, 1(6): 814–825
Dong X L, Huang Y D. Synthesis of 2,5-bis(4-alkoxy-phenyl)-1,3,4-oxadiazoles. Chemical Industry and Engineering, 2013, 30(2): 36–39 (in Chinese)
Huang Y D, Dong X L, Zhang L L, Chai W, Chang J Y. Structureproperty correlation of benzoyl thiourea derivatives as organogelators. Journal of Molecular Structure, 2013, 1031: 43–48
Eldridge J E, Ferry J D. Studies of the cross-linking process in gelatin gels. III. Dependence of melting point on concentration and molecular weight. Journal of Physical Chemistry, 1954, 58(11): 992–995
Huang Y D, Tu W, Yuan Y Q, Fan D L. Novel organogelators based on pyrazine-2,5-dicarboxylic acid derivatives and their mesomorphic behaviors. Tetrahedron, 2014, 70(6): 1274–1282
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sun, Z., Dong, X., Zhai, Y. et al. 2,5-Dialkoxylphenyl-1,3,4-oxadiazoles as efficient organogelators and their self-assembling property. Front. Chem. Sci. Eng. 8, 219–224 (2014). https://doi.org/10.1007/s11705-014-1418-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11705-014-1418-x