Abstract
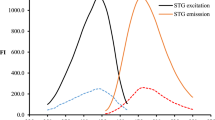
Alogliptin benzoate (ALG) is a dipeptidyl peptidase-4 inhibitor used to treat type 2 diabetes mellitus. In this study, a spectrofluorimetric method was developed for the derivatization of ALG to develop a fluorescent derivative. The derivatization reaction was carried out by reacting ALG with ninhydrin and phenylacetaldehyde in Teorell and Stenhagen buffer at pH 6.6. The reaction product was a fluorescent pyrrolidone derivative that was observed at λex of 385 nm and λem of 475 nm. The effects of various experimental conditions on the derivatization reaction were investigated. The optimal conditions were found to be a reaction time of 15 min, a temperature of 80 °C, and a concentration of ninhydrin and phenylacetaldehyde of 1.4 mg mL−1 and 0.028% v/v, respectively. A calibration plot was constructed in the concentration range of 20–460 ng mL−1. The calibration curve had a high correlation coefficient of 0.9991 and a low detection limit of 6.57 ng mL−1. The eco-scale penalty points for the derivatization method were calculated to be 86. This indicates that the method is environmentally friendly and has a low risk of generating harmful waste. The derivatization method described in this study provides a sensitive and green method for the quantification of ALG in pure form, dosage form, spiked and real rat plasma.







Similar content being viewed by others
References
Abdel-Lateef MA, Almahri A (2022) Spectrofluorimetric determination of α-difluoromethylornithine through condensation with ninhydrin and phenylacetaldehyde: application to pharmaceutical cream and spiked urine samples. Chem Pap 76(2):741–748. https://doi.org/10.1007/s11696-021-01894-3
Abouzid MR, Ali K, Elkhawas I, Elshafei SM (2022) An overview of diabetes mellitus in Egypt and the significance of integrating preventive cardiology in diabetes management. Cureus 14(7):e27066
Almahri A et al (2021) Resonance Rayleigh scattering and spectrofluorimetric approaches for the selective determination of rupatadine using erythrosin B as a probe: application to content uniformity test. Luminescence 36(3):651–657
Aref HA, Hammad SF, Darwish KM, Elgawish MS (2020) Novel spectrofluorimetric quantification of Alogliptin benzoate in biofluids exploiting its interaction with 4-chloro-7-nitrobenzofurazan. Luminescence 35(2):284–291
Attia KAM et al (2022) Construction and application of highly sensitive spinel nanocrystalline zinc chromite decorated multiwalled carbon nanotube modified carbon paste electrode (ZnCr2O4@MWCNTs/CPE) for electrochemical determination of Alogliptin benzoate in bulk and its dosag. RSC Adv 12(30):19133–19143
Binkadem MS et al (2023) Development of sٍٍensitive spectrofluorimetric methods for determining netilmicin based on selective condensation reactions of its amine moiety with each acetylacetone/formaldehyde and ninhydrin/phenylacetaldehyde reagents. Spectrochim Acta Part A Mol Biomol Spectrosc 299:122839
Butnariu M et al (2021) Analytical and in silico study of the inclusion complexes between tropane alkaloids atropine and scopolamine with cyclodextrins. Chem Pap 75(10):5523–5533. https://doi.org/10.1007/s11696-021-01742-4
Butnariu M, Sarac I, Samfira I (2020) Spectrophotometric and chromatographic strategies for exploring of the nanostructure pharmaceutical formulations which contains testosterone undecanoate. Sci Rep 10(1):1–10
BernardoSilvano De et al (1974) Studies on the reaction of fluorescamine with primary amines. Arch Biochem Biophys 163(1):390–399
Derayea SM, Attia TZ, Elnady M (2018) Development of spectrofluorimetric method for determination of certain antiepileptic drugs through condensation with ninhydrin and phenyl acetaldehyde. Spectrochim Acta Part A Mol Biomol Spectrosc 204:48–54. https://doi.org/10.1016/j.saa.2018.06.027
Derayea SM et al (2020) Spectrofluorometric determination of Alogliptin an antidiabetic drug in pure and tablet form using fluorescamine, a fluorogenic agent: application to content uniformity test. Luminescence 35(7):1028–1035
Deshpande PB, Butle SR (2017) Stability indicating high performance thin layer chromatographic determination of Alogliptin benzoate as bulk drug and in tablet dosage form. Eurasian J Anal Chem 12(4):325–335
El-Bagary RI, Elkady EF, Ayoub BM (2012) Liquid chromatographic determination of Alogliptin in bulk and in its pharmaceutical preparation. Int J Biomed Sci 8(3):215–218
Fejős I et al (2014) Separation of Alogliptin enantiomers in cyclodextrin-modified capillary electrophoresis: a validated method. Electrophoresis 35(19):2885–2891
Gałuszka A, Migaszewski ZM, Konieczka P, Namieśnik J (2012) Analytical eco-scale for assessing the greenness of analytical procedures. TrAC Trends Anal Chem 37:61–72
Gonzalez-Oñate A, Quevedo R (2020) Ninhydrin reaction with phenylethylamine: unavoidable by-products. J Chem Sci 132(1):1–6
Guideline ICH, Tripartite H (2005) Validation of analytical procedures: text and methodology. Q2 (R1) 1(20):5
Hamad AA, Derayea SM (2023) A novel and unusual utility of the cardiosintol drug as a fluoro-prober in the amendment of a highly fluorescent module for determining the non-fluorescent N-acetylcysteine drug. Spectrochim Acta Part A Mol Biomol Spectrosc 293:122460. https://doi.org/10.1016/j.saa.2023.122460
Hegazi R, El-Gamal M, Abdel-Hady N, Hamdy O (2015) Epidemiology of and risk factors for type 2 diabetes in Egypt. Ann Glob Health 81(6):814–820
ICH (2022) “ICH guideline Q2(R2) on validation of analytical procedures 2(0)
Lamie NT, Mahrouse MA (2018) Smart spectrophotometric methods based on normalized spectra for simultaneous determination of Alogliptin and metformin in their combined tablets. Spectrochim Acta Part A Mol Biomol Spectrosc 204:743–747. https://doi.org/10.1016/j.saa.2018.07.004
Lee B et al (2008) Pharmacokinetic, pharmacodynamic, and efficacy profiles of Alogliptin, a novel inhibitor of dipeptidyl peptidase-4, in rats, dogs, and monkeys. Eur J Pharmacol 589(1–3):306–314
Manidipa D, Ashutosh KS, Seshagiri RJVLN, Gowri SD (2015) New validated stability indicating RP-HPLC method for simultaneous estimation of metformin and Alogliptin in human plasma. J Chromatogr Sep Tech 06(06):6–11
Mostafa IM, Derayea SM, Nagy DM, Omar MA (2018) An experimental ninhydrin design approach for the sensitive spectrofluorimetric assay of milnacipran in human urine and plasma. Spectrochim Acta Part A Mol Biomol Spectrosc 205:292–297
Omar MA, Hammad MA, Nagy DM, Aly AA (2015) Development of spectrofluorimetric method for determination of certain aminoglycoside drugs in dosage forms and human plasma through condensation with ninhydrin and phenyl acetaldehyde. Spectrochim Acta Part A Mol Biomol Spectrosc 136:1760–1766
Raja PMV, Barron AR (1934) Physical methods in chemistry. Nature 134(3384):366–367
Saeedi P et al (2019) Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: results from the international diabetes federation diabetes Atlas, 9th edition. Diabetes Res Clin Pract 157:107843
Salim MM et al (2023) Using of eosin Y as a facile fluorescence probe in Alogliptin estimation: application to tablet dosage forms and content uniformity testing. Spectrochim Acta Part A Mol Biomol Spectrosc 285:121919
Samejima K, Dairman W, Udenfriend S (1971) Condensation of ninhydrin with aldehydes and primary amines to yield highly fluorescent ternary products: I. studies on the mechanism of the reaction and some characteristics of the condensation product. Anal Biochem 42(1):222–236
Sharma K, Parle A (2015a) 11-Development and validation of HPTLC method for simultaneous estimation of Alogliptin benzoate and pioglitazone hydrochloride in bulk drugs and combined dosage forms. Int J Pharma Res 4(11):35–42
Sharma K, Parle A (2015b) Development and validation of HPTLC method for estimation of Alogliptin benzoate in bulk drugs and tablet dosage forms. Int Bull Drug Res 5(8):81–89
Sunil Kumar AVVNK, Reddy TV, Sekharan CB (2017) Spectrophotometric determination of Alogliptin in bulk and tablet dosage form using bromate-bromide mixture as brominating agent. Karbala Int J Mod Sci 3(1):8–17. https://doi.org/10.1016/j.kijoms.2016.12.002
Tammam AS, Gahlan AA, Taher MA, Haredy AM (2022) Hantzsch condensation reaction as a spectrofluorometric method for determination of Alogliptin, an antidiabetic drug, in pure form, tablet form, and human and rat plasma. Luminescence 37(4):543–550
Vinyas M et al (2016) Analytical method development and validation of Alogliptin by RP-HPLC method. Res J Pharm Technol 9(7):775–778
Zaghary WA, Mowaka S, Hassan MA, Ayoub BM (2017) Comparative study between different simple methods manipulating ratio spectra for the analysis of Alogliptin and metformin co-formulated with highly different concentrations. Spectrochim Acta Part A Mol Biomol Spectrosc 186:23–28
Zhang K, Ma P, Jing W, Zhang X (2015) A developed HPLC method for the determination of Alogliptin benzoate and its potential impurities in bulk drug and tablets. Asian J Pharm Sci 10(2):152–158. https://doi.org/10.1016/j.ajps.2015.01.001
Zwir-Ferenc A, Biziuk M (2006) Solid phase extraction technique—trends, opportunities and applications. Pol J Environ Stud 15(5):677–690
Acknowledgements
None.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
There are no conflicts to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
El-Gizawy, S.M., Atia, N.N., Rushdy, D.H. et al. Spectrofluorimetric determination of Alogliptin benzoate through condensation with ninhydrin and phenylacetaldehyde: application to dosage form and rat plasma. Chem. Pap. (2024). https://doi.org/10.1007/s11696-024-03451-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11696-024-03451-0