Abstract
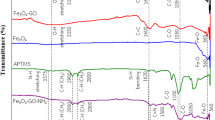
An eco-friendly magnetic nanoparticle (MNPs)-based stir rod-assisted dispersive solid-phase extraction (SRMDSPE) method was proposed for the removal and extraction of Cr (VI) preceding its spectrophotometric determination. The magnetic melamine‐functionalized chitosan-modified graphene oxide (MCMGO) nanocomposites were synthesized as a biocompatible, effective adsorbent with admirable adsorption capacity, great magnetic property and excellent dispersion ability for the adsorption of the Cr (VI) ion. The prepared adsorbent was characterized by XRD, FTIR, TGA, VSM, EDX, and SEM. Different parameters affecting removal and microextraction efficiencies such as sample volume, pH, interferences, volume and type of eluent, and adsorbent dosage were investigated and optimized. The result of isotherm shows that Cr (VI) adsorption followed the Freundlich model. Thermodynamic parameters (i.e., change in the free energy (ΔG0), the enthalpy (ΔH0), and the entropy (ΔS0)) were also evaluated. The overall adsorption process was endothermic and spontaneous. The sorbent elution with ability and reusability was used in dispersive solid-phase extraction. Under optimal conditions, the extraction efficiency was 78.0%, and the enrichment factor was 31.19. The SRMDSPE was successfully quantified in the 0.05–0.5 mg L−1 (R2 = 0.9993) with a detection limit of 0.015 mg L−1. The proposed method was applied to leather wastewater, Tabriz, and some surface waters. The percentage of recoveries for spiked real samples were in the range of 91–101%, and the percentage of relative standard deviations were 2–6%.








Similar content being viewed by others
References
Anush S, Chandan H, Gayathri B, Manju N, Vishalakshi B, Kalluraya B (2020) Graphene oxide functionalized chitosan-magnetite nanocomposite for removal of Cu (II) and Cr (VI) from waste water. Int J Biol Macromol 164:4391–4402
Arain MB, Ali I, Yilmaz E, Soylak M (2018) Nanomaterial’s based chromium speciation in environmental samples: a review. TrAC Trends Anal Chem 103:44–55
Bandara PC, Nadres ET, Rodrigues DF (2019) Use of response surface methodology to develop and optimize the composition of a chitosan–polyethyleneimine–graphene oxide nanocomposite membrane coating to more effectively remove Cr (VI) and Cu (II) from water. ACS Appl Mater Interfaces 11(19):17784–17795
Béni Á, Karosi R, Posta J (2007) Speciation of hexavalent chromium in waters by liquid–liquid extraction and GFAAS determination. Microchem J 85(1):103–108
Borai E, El-Sofany E, Abdel-Halim A, Soliman A (2002) Speciation of hexavalent chromium in atmospheric particulate samples by selective extraction and ion chromatographic determination. TrAC Trends Anal Chem 21(11):741–745
Büyüktiryaki S, Keçili R, Hussain CM (2020) Functionalized nanomaterials in dispersive solid phase extraction: advances & prospects. TrAC Trends Anal Chem 127:115893
Chen B-H, Jiang S-J, Sahayam A (2020) Determination of Cr (VI) in rice using ion chromatography inductively coupled plasma mass spectrometry. Food Chem 324:126698
Directive C (1998) On the quality of water intended for human consumption. Off J Eur Commun 330:32–54
Elci L, Divrikli U, Akdogan A, Hol A, Cetin A, Soylak M (2010) Selective extraction of chromium(VI) using a leaching procedure with sodium carbonate from some plant leaves, soil and sediment samples. J Hazard Mater 173(1):778–782
Freundlich H (1907) Über die adsorption in Lösungen. Z Phys Chem 57U(1):385–470
Ge H, Du J (2020) Selective adsorption of Pb (II) and Hg (II) on melamine-grafted chitosan. Int J Biol Macromol 162:1880–1887
Jamroz E, Kocot K, Zawisza B, Talik E, Gagor A, Sitko R (2019) A green analytical method for ultratrace determination of hexavalent chromium ions based on micro-solid phase extraction using amino-silanized cellulose membranes. Microchem J 149:104060
Karimi-Maleh H, Ayati A, Ghanbari S, Orooji Y, Tanhaei B, Karimi F, Alizadeh M, Rouhi J, Fu L, Sillanpää M (2021) Recent advances in removal techniques of Cr (VI) toxic ion from aqueous solution: a comprehensive review. J Mol Liq 329:115062
Khan A, Ali N, Bilal M, Malik S, Badshah S, Iqbal H (2019) Engineering functionalized chitosan-based sorbent material: characterization and sorption of toxic elements. Appl Sci 9(23):5138
Leite VDSA, de Jesus BGL, de Oliveira Duarte VG, Constantino VRL, Izumi CMS, Tronto J, Pinto FG (2019) Determination of chromium (VI) by dispersive solid-phase extraction using dissolvable Zn–Al layered double hydroxide intercalated with l-Alanine as adsorbent. Microchem J 146:650–657
Liu W, Zheng J, Ou X, Liu X, Song Y, Tian C, Rong W, Shi Z, Dang Z, Lin Z (2018) Effective extraction of Cr (VI) from hazardous gypsum sludge via controlling the phase transformation and chromium species. Environ Sci Technol 52(22):13336–13342
Marcano DC, Kosynkin DV, Berlin JM, Sinitskii A, Sun Z, Slesarev A, Alemany LB, Lu W, Tour JM (2010) Improved synthesis of graphene oxide. ACS Nano 4(8):4806–4814
Montoro-Leal P, García-Mesa J, Cordero MS, Guerrero ML, Alonso EV (2020) Magnetic dispersive solid phase extraction for simultaneous enrichment of cadmium and lead in environmental water samples. Microchem J 155:104796
OEHHA C (2011) Public health goal for hexavalent chromium (Cr VI) in drinking water, California Environmental Protection Agency, Office of Environmental Health Hazard Assessment
Padmavathy K, Madhu G, Haseena P (2016) A study on effects of pH, adsorbent dosage, time, initial concentration and adsorption isotherm study for the removal of hexavalent chromium (Cr (VI)) from wastewater by magnetite nanoparticles. Proc Technol 24:585–594
Pourmohammad M, Faraji M, Jafarinejad S (2020) Extraction of chromium (VI) in water samples by dispersive liquid–liquid microextraction based on deep eutectic solvent and determination by UV–Vis spectrophotometry. Int J Environ Anal Chem 100(10):1146–1159
Séby F, Vacchina V (2018) Critical assessment of hexavalent chromium species from different solid environmental, industrial and food matrices. TrAC Trends Anal Chem 104:54–68
Sereshti H, Amini F, Najarzadekan H (2015) Electrospun polyethylene terephthalate (PET) nanofibers as a new adsorbent for micro-solid phase extraction of chromium(vi) in environmental water samples. RSC Adv 5(108):89195–89203
Sereshti H, Vasheghani Farahani M, Baghdadi M (2016) Trace determination of chromium(VI) in environmental water samples using innovative thermally reduced graphene (TRG) modified SiO2 adsorbent for solid phase extraction and UV–vis spectrophotometry. Talanta 146:662–669
Shi Y, Xiong D, Zhao Y, Li T, Zhang K, Fan J (2020) Highly efficient extraction/separation of Cr (VI) by a new family of hydrophobic deep eutectic solvents. Chemosphere 241:125082
Sobhi HR, Azadikhah E, Behbahani M, Esrafili A, Ghambarian M (2018) Application of a surfactant-assisted dispersive liquid-liquid microextraction method along with central composite design for micro-volume based spectrophotometric determination of low level of Cr (VI) ions in aquatic samples. Spectrochim Acta Part A Mol Biomol Spectrosc 202:36–40
Subedi N, Lähde A, Abu-Danso E, Iqbal J, Bhatnagar A (2019) A comparative study of magnetic chitosan (Chi@ Fe3O4) and graphene oxide modified magnetic chitosan (Chi@ Fe3O4GO) nanocomposites for efficient removal of Cr (VI) from water. Int J Biol Macromol 137:948–959
University of California, Berkeley (2008). Introduction. Psychology 1: Fall 2007—Introduction to the principal areas, problems, and concepts of psychology. UCBerkeley
Vakili M, Deng S, Cagnetta G, Wang W, Meng P, Liu D, Yu G (2019) Regeneration of chitosan-based adsorbents used in heavy metal adsorption: a review. Sep Purif Technol 224:373–387
Water ID (2011) Responses to major comments on technical support document public health goal for hexavalent chromium (Cr VI)
Wu Z-C, Wang Z-Z, Liu J, Yin J-H, Kuang S-P (2015) A new porous magnetic chitosan modified by melamine for fast and efficient adsorption of Cu(II) ions. Int J Biol Macromol 81:838–846
Yousefi SM, Shemirani F (2017) Carbon nanotube-based magnetic bucky gels in developing dispersive solid-phase extraction: application in rapid speciation analysis of Cr (VI) and Cr (III) in water samples. Int J Environ Anal Chem 97(11):1065–1079
Zhu Q-Y, Zhao L-Y, Sheng D, Chen Y-J, Hu X, Lian H-Z, Mao L, Cui X-B (2019) Speciation analysis of chromium by carboxylic group functionalized mesoporous silica with inductively coupled plasma mass spectrometry. Talanta 195:173–180
Acknowledgements
This project is supported by research grant of University of Tabriz (Grant Number: 97/3/7-890).
Author information
Authors and Affiliations
Contributions
VB was involved in the investigation and writing—original draft; AN was involved in reviewing and editing and supervision; SSA contributed to the supervision and resources, MS assisted in the supervision, conceptualization, reviewing and editing; ZZ performed the supervision, conceptualization, reviewing and editing.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Bagheri, V., Naseri, A., Sajedi-Amin, S. et al. Using Fe3O4-graphene oxide-modified chitosan with melamine magnetic nanocomposite in the removal and magnetic dispersive solid-phase microextraction of Cr (VI) ion in aquatic samples. Chem. Pap. 78, 381–396 (2024). https://doi.org/10.1007/s11696-023-03096-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11696-023-03096-5