Abstract
Apple pomace is a waste stream produced by fruit processing industry millions of tons annually. It is rich in carbohydrates making it a potential feedstock for the production of 5-hydroxymethylfurfural (HMF), one of the most valuable platform chemicals. In this work, the conversion of apple pomace carbohydrates to HMF was studied in a choline chloride:glycolic acid (1:3) deep eutectic solvent. To prevent undesired side reactions of HMF methyl isobutyl ketone was added to the reaction system as an extractive phase. The effect of reaction conditions, i.e., the amount of water added to the reaction system, the presence of Lewis acid co-catalyst, as well as the reaction temperature and time, on HMF yield were studied. The highest total HMF yield (44.5%) was achieved at 110 °C in 10 min with 15 wt% H2O, and 0.01 g CrCl3 as co-catalyst. Without the co-catalyst, the highest achieved HMF yield was 37.3% (120 °C, 20 min, 15 m% H2O). The results indicated that apple pomace can be used as the feedstock for HMF production but the reaction procedure, especially the extraction process of HMF from deep eutectic solvent needs to be studied further.
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
With energy crisis at hand and due to the concerns of environmental deterioration, it is important to find renewable alternatives to fossil resources. The production of biomass-based platform chemicals such as HMF has thusly received much attention during the last decades (Mika et al. 2018). HMF is a versatile chemical, which can be converted into various commercial chemicals used e.g., as biofuels, solvents, or monomers for plastics. HMF is produced by acid catalyzed dehydration reaction of C6-sugars, such as glucose or fructose. Its production has been extensively studied by using various feedstocks as well as different reaction media like aqueous and organic solvents, biphasic systems, sub or supercritical fluids and ionic liquids. Numerous review articles, which summarize comprehensively the various HMF production methods, yields and proposed reactions routes have been written during the last decade, and readers are encouraged to refer those for more detailed information (Teong et al. 2014; Yu and Tsang 2017; Mika et al. 2018; Zhao et al. 2021).
Deep eutectic solvents (DESs) are liquid, homogeneous mixtures composed of a hydrogen bond donor (HBD) and a hydrogen bond acceptor (HBA). They are prepared simply by mixing and gently heating together the components. The ratio of the components depends on the DES to be prepared. DESs are considered as easily prepared, low-cost green solvents, which are non-toxic and have high biodegradability (Abbott et al. 2004). Even though DESs are a quite new group of solvents, they have been utilized in biomass pre-treatment as well as in catalytic conversion of carbohydrates to HMF (Tang et al. 2017; Chen et al. 2022). DESs used in conversion reactions are often composed of choline chloride as the HBA and carboxylic acid e.g., oxalic acid, malic acid, or citric acid as the HBD. (Tang et al. 2017).
Apple pomace is a fruit processing industry waste stream; a press cake formed when apples are pressed e.g., for juice or cider production (Kennedy et al. 1999). The annual production of apples worldwide is ca. 70 million tons, and considerable amount of it is used to produce apple products (Massias et al. 2015). The quantity of apple pomace formed varies from 25 to 40% of the total amount of fruits processed, and depends e.g., on the fruit variety and the apple pressing technology (Rachana and Gupta 2010; Wilczyński et al. 2019). Apple pomace is rich in valuable components such a carbohydrates and nutrients, so discarding it is waste of resources. In addition, fresh apple pomace is biodegradable and perishable meaning that disposal of it as waste can cause serious environmental problems (Perussello et al. 2017). Since apple pomace has high carbohydrate content, various amounts like 48–84% has been reported in literature (Dhillon et al. 2013; Perussello et al. 2017), it could be a feasible feedstock for HMF production.
The aim of this work was to study the conversion of apple pomace to HMF in an environmentally friendly choline chloride:glycolic acid (1:3) DES, which also acted as a Bronsted acid catalyst for the reaction. The selection of DES was based on a recent study of Rusanen et al. in which it was found that the choline chloride:glycolic acid (1:3) DES formed fast in relatively low temperature and remained liquid in room temperature (Rusanen et al. 2021). Reactions were performed in biphasic system, which consisted of the DES phase and the extracting MIBK phase to extract the formed HMF from the DES during the reaction. The purpose was to study the effect of the reaction conditions, i.e., reaction temperature and time, and the amount of water added to the reaction system, on HMF yield. Also, the effect of Lewis acid co-catalyst on the HMF yield was studied.
Experimental
Materials
Choline chloride (> 98%, TCI), glycolic acid (98%, Fluorochem), methyl isobutyl ketone (99.5%, Acros organics), glucose (> 99%, Sigma-Aldrich), fructose (> 99%, BDH Chemicals), AlCl3·6 H2O (99%, Alfa Aesar), CrCl3·6H2O (98%, Alfa Aesar), methanol (99.9%, Honeywell), ethyl acetate (< 99.7%, Honeywell), furfural (98%, Alfa Aesar), HMF (98%, Acros Organics), acetone (99.8%, VWR Chemicals), acetic acid (99.8%, FF chemicals), NaOH (99.8%, VWR Chemicals), NaClO2 (80%, Honeywell), dimethyl sulfone (100%, Sigma-Aldrich), CDCl3 (99.8%, Euriso-top), and deuterium oxide (99.96%, VWR Chemicals) were used as received, without further purification. Choline chloride and glycolic acid were stored in a desiccator to prevent them from absorbing moisture. Water used in the reactions was Elix water.
Apple pomace was received from a local juice pressing facility, Marjarannikko (Tupos, Finland), and stored in a freezer. Before use, the pomace was dried in an oven at 45 °C until constant weight, and ground to a fine powder with a coffee grinder. An estimate of the composition of dry apple pomace was obtained by using known methods (Rusanen et al. 2019). The ash content determination was made for unextracted apple pomace but for other determinations extracted pomace was used. All analyses were performed in duplicate.
Conversion reaction of apple pomace in deep eutectic solvent
Each conversion reaction was begun by preparing the DES. Choline chloride (0.949 g, 6.8 mmol) and glycolic acid (1.551 g, 20.4 mmol) were weighted into a microwave reactor tube (Biotage, size 2–5 ml). The tube was closed and heated with vigorous stirring in an oil bath (70 °C) until a clear, colorless liquid was obtained. (Rusanen et al. 2021) Analysis of the prepared DES by thermogravimetric analysis and 1H NMR was published in our previous article and can be found there (Rusanen et al. 2021). Next, water (0–20 wt%, based on the mass of the DES), apple pomace (0.1 g) and Lewis acid (0.01; 0.05 or 0.1 g) were added into the tube and mixed well with the DES. Finally, MIBK (2 ml) was added as the second, extracting phase into the tube. Reaction mixture was heated in a microwave reactor (Biotage Initiator) at 150–170 °C for 10–30 min.
After the reaction, the reactor tube was let to cool to room temperature. MIBK layer was separated with a syringe and a needle. The DES layer was extracted three times with 2 ml of MIBK. All the MIBK layers were combined, and the solution was filtered with 0.45 μm nylon syringe filter. For the product analysis a 200-μL sample was taken to a vial from the MIBK solution, and it was diluted with 800 μL methanol. All conversion reactions were performed in duplicate.
Analysis of products with liquid chromatography
HMF and furfural were analyzed with Waters 2695 separation module combined with Waters 996 photodiode array (PDA) detector. Atlantis T3 (3 μm, 4.6 × 150 mm) column was used for the analysis of the organic phases of the reactions, and Atlantis dC18 column (5 μm, 4.6 × 150 mm) was used for the analysis of DES phases of the reactions. Eluent was a 90:10 mixture of water (0.1% TFA) and methanol (0.1% TFA); the flow rate was 1 mL/min. The column temperature was kept constant at 30 °C. UV detection for HMF was performed with a wavelength of 284 nm, while for furfural, 277 nm was used. Calibrations were performed using commercial HMF, and all samples were analyzed in duplicate. The HMF yields are expressed as the ratio of HMF determined from the MIBK or DES phase to the initial holocellulose (hemicellulose + cellulose) content of the apple pomace fed to the reaction.
Results and discussion
Apple pomace characterization
Apple pomace was subjected to compositional analysis before use to get an estimate of its composition. The results of the analysis are presented in Table 1. The presented lignin amount contains both the soluble (3 wt%) and insoluble lignin (11 wt%). The determined holocellulose amount (21%) was used as the initial holocellulose content when the HMF yields were calculated.
The determined apple pomace composition is somewhat in line with literature. In the study of Melikoglu et al. (2019) the total lignin content of the pomace was found to be 22.5 wt%, α-cellulose content 32.5 wt% and hemicellulose content 29 wt% (Melikoglu et al. 2019). Szymanska-Chargot et al. (2015) determined the α-cellulose content of apple fruit to be 12–25 wt% depending on the development stage and variety of the fruit, while hemicellulose content was 23–40 wt% (Szymanska-Chargot et al. 2015). Villas-Boas et al. (2004) on the other hand found the α-cellulose content of apple pomace and lignin to be 7.4 wt% and 23.3 wt%, respectively (Villas-Boas et al. 2004). The analysis methods used in literature and this work vary between studies, also the variety and development stage of the apples cause variation to the determined compositions; Gassara et al. (2012) also mentioned that juice processing facilities use variable amounts of rice husk to facilitate the juice extraction, which can distort the composition analyses (Gassara et al. 2012).
Conversion of apple pomace to 5-hydroxymethylfurfural in deep eutectic solvent
The DES (1:3 choline chloride:glycolic acid) and the reaction conditions used in this study were selected based on literature (Rusanen et al. 2021) and some preliminary experiments (data not shown). The formation of DES took 5 min, and it was a clear, colorless liquid with viscosity similar to water. Water content of DES (before addition of water) was determined in our previous study, which used the same reagents, and was found to be 3.2% (Rusanen et al. 2021). Water (when used) and DES dissolved easily to one another. The apple pomace, dry powder with orange color (Fig. 1), mixed also easily with DES, but no dissolution was observed. After the reaction, the color of DES phase varied, depending on the reaction conditions, from orange to dark brown. The color of MIBK phase varied from yellow to dark orange. The DES and MIBK phases separated well from each other after the reaction, making the removal of MIBK phase easy. After each reaction, the DES phase contained reddish to dark brown solids indicating that the pomace did not dissolve in the DES during the reaction, and that some humin (undesirable side products derived from HMF (Tsilomelekis et al. 2016)) formation had occurred especially when high (150–170 °C) reaction temperature was used. The amount of HMF in MIBK phase of each reaction was determined with HPLC. The HPLC chromatograms indicated that the MIBK phases contained several other compounds besides HMF, only furfural was identified though. Common HMF degradation product, levulinic acid, was not detected from the MIBK phase samples.
Effect of water on 5-hydroxymethylfurfural formation
The effect of water on HMF yield was studied by adding 0–20 wt% water to DES. Based on the preliminary reactions, the reaction temperature and time were selected to be 140 °C and 20 min, respectively. The results are presented in Fig. 2.
Water was found to be beneficial to conversion reaction. The lowest yield, 5.7% was obtained when no water was added to the reaction. The HMF yield increased with increasing amount of water, and highest yield, 16.7% was achieved with 15 wt% of added water (based in the mass of the DES). Higher amount of water did not seem to increase the HMF yield further, so 15 wt% was selected to be used as the water amount in subsequent reactions. Similar effect of water on HMF yield has been observed in other studies as well. Zuo et al. (2021a) studied the glucose conversion to HMF in two deep eutectic solvents, glucose:choline chloride (1:3) and water:glucose:choline chloride (1:1:3). In their study, the HMF yield increased from 20 to 28.8%, when DES was changed from glucose:choline chloride (1:3) to water:glucose:choline chloride (1:1:3) and other reaction conditions were kept constant (reaction temperature 130 °C, reaction time 4 h and the amount of AlCl3 catalyst 2 wt%). (Zuo et al. 2021a) In the study of Rusanen et al. (2021) the HMF yield increased from 3 to 7 wt%, when the amount water was increased (Rusanen et al. 2021). It has been suggested in the literature that the increase in HMF yields due to the increased amount of water could be related to the decreased viscosity of the system, which would allow the better heat and mass transfer. Increased amount of water in the system could also increase the solubility of the feedstock sugars during the reaction enhancing thusly their reactivity. (Qi et al. 2012; Zuo et al. 2021b) It should be noted though that adding too much water in DES may cause the interactions between the DES components to cease, in which case DES becomes an aqueous solution (Hammond et al. 2017; Zuo et al. 2021b). Rusanen et al. (2021) determined that for 1:3 choline chloride:glycolic acid DES as much as 31 wt% of water could be added without rupturing the hydrogen bonding structure of DES. (Rusanen et al. 2021).
Effect of reaction temperature and time on 5-hydroxymethylfurfural formation
The effect of reaction temperature on HMF yield was studied by varying the temperature from 120 to 170 °C. The amount of water and the reaction time were kept constant, 15 wt% and 20 min, respectively. The results are presented in Fig. 3.
Based on the results, the reaction temperature did not seem to have as high effect on the HMF yield as the amount of water. Highest yield, 17.1%, was obtained already at 130 °C and the yield decreased clearly when the temperature was 150 °C or higher. Reaction temperature of 130 °C, compared to higher temperatures, is beneficial from the choline chloride:glycolic acid (1:3) DES point of view. Rusanen et al. (2021) as well as Rodriguez-Rodriguez et al. (2019) showed with their thermogravimetric analyses that this particular DES started to decompose already at 150 °C albeit the decomposition was still minor at that temperature (Rodriguez-Rodriguez et al. 2019; Rusanen et al. 2021).
The effect of the reaction time on HMF yield was studied by varying the time from 10 to 30 min. Since 130 °C was found to produce the highest HMF yield, it was used as the reaction temperature; the amount of water was kept as 15 wt%. The results are presented in Fig. 4.
The reaction time did not seem to have much effect on the HMF yield. 30 min reaction time gave the highest HMF yield (17.6%) but there was more deviation between the results of the parallel reactions. Therefore 20 min was selected to be used as the reaction time in subsequent reactions. The studied reaction temperatures and times for HMF synthesis reported in literature vary greatly, from 80 to 200 °C, and from minutes to several hours (Mika et al. 2018), respectively, since they usually depend on each other as well as on the used feedstock, catalyst(s), and reaction medium.
The effect of Lewis acid co-catalyst on 5-hydroxymethylfurfural formation
The conversion of C6 monosaccharides such as glucose to HMF proceeds via isomerization phase; in case of glucose the isomerization product is fructose. It has been shown in previous studies that the isomerization phase can be catalyzed with Lewis acid, usually metal chloride, and adding Lewis acid to the reaction system often improves the HMF yield. In most of the research articles the isomerization has been proposed to proceed through enolization pathway, which starts with the formation of a glucose-catalyst complex. The metal of the metal chloride coordinates with two oxygen atoms of the glucose forming five-membered chelated structure from which fructose forms after enolization (Hu et al. 2009; Choudhary et al. 2013; Istasse and Richel 2020). A reaction route for glucose to fructose isomerization based on the study by Hu et al. (2009) is presented in Fig. 5. Detailed descriptions of various studied and proposed reaction routes for conversion of biomass to HMF are summarized in e.g., the review of Zhao et al. (2021). Therefore, it was decided to study, if the addition of Lewis acid as co-catalyst to the reaction mixture would increase the HMF yield. The studied Lewis acids were CrCl3 and AlCl3, and they were used as hexahydrates. Both acids were studied with three amounts (0.1; 0.05 and 0.01 g). Reaction temperature, time, and the amount of water were the previously determined 130 °C, 20 min and 15 wt%, respectively. The results are presented in Fig. 6.
The proposed reaction route to for the Lewis acid, i.e., metal chloride, catalyzed isomerization of glucose to fructose (Hu et al. 2009)
The effect of different amounts Lewis acid AlCl3·6H2O or CrCl3·6H2O on the HMF yield from apple pomace in deep eutectic solvent. The given amounts of Lewis acids mean the amount of AlCl3 or CrCl3, not the hexahydrate. Reaction conditions: 130 °C, 20 min, water 15 wt%, 0.1 g apple pomace. The results are presented as the average of two parallel reactions, and the error bars denote the deviation of the parallel results
Based on the results with both Lewis acids the lowest amount of acid gave the highest HMF yield. Also, it seemed that CrCl3 was a better choice as co-catalyst than AlCl3, which is in line with the study of Zuo et al. (2021a). In their article Zuo et al. suggested that AlCl3 had excessive acidity which led to the formation of humins and other small molecules instead of HMF (Zuo et al. 2021a). However, neither of the acids improved the HMF yield compared to reactions performed without the Lewis acid co-catalyst. Since the reactions with Lewis acids were performed in constant reaction conditions, it was thought that perhaps those conditions were not optimal for the Lewis acid co-catalyzed reaction. Therefore, reactions with CrCl3·6H2O were decided to perform with different reaction temperatures (90–150 °C), and also with a shorter reaction time (10 min). The results are presented in Fig. 7.
The effect of reaction temperature and time on the HMF yield from apple pomace in deep eutectic solvent, when CrCl3·6H2O was used as the co-catalyst. Reaction conditions: water 15 wt%, 0.1 g apple pomace. The results are presented as the average of two parallel reactions, and the error bars denote the deviation of the parallel results
The results indicated that changing the reaction temperature or time did not improve the HMF yield markedly. It seemed to be slightly more beneficial to use a lower reaction temperature and longer time (110 °C and 20 min, respectively) than higher temperature and shorter time (120 °C and 10 min, respectively); however, the difference was small compared to deviation between the results of the parallel reactions. Based on the results so far it was thusly concluded that in this study the Lewis acid as co-catalyst did not enhance the apple pomace conversion reaction to HMF.
The efficiency of the extraction process of 5-hydroxymethylfurfural
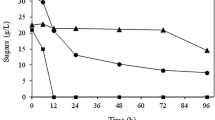
The HMF yields obtained with previous reactions were quite modest, 17.6% at best. Rusanen et al. (2021) noticed in their study that they were able to extract only 45% of the HMF dissolved in choline chloride:glycolic acid (1:3) DES with MIBK (3 × 2 ml) (Rusanen et al. 2021). Hence it was thought that perhaps the extraction process of HMF from DES phase with MIBK was not sufficient in this study either. Since only the MIBK phases from the reactions had been analyzed in this study so far, it was decided to also analyze some of the DES phases. The results are presented in Fig. 8.
The HMF yields achieved from selected conversion reactions of apple pomace in deep eutectic solvent, when both the MIBK and the DES phase of the reaction system were analyzed: reactions performed a with variable amounts of water (140 °C, 20 min); b in different reaction temperatures (20 min, 15 wt% water); c with different reaction times (130 °C, 15 wt% water); d with variable reaction temperatures and times with CrCl3·6H2O added as the co-catalyst (amount corresponding 0.01 g of CrCl3)
Indeed, all the analyzed DES phases contained considerable amounts of HMF; in most of the cases the amount of HMF in DES phase was even higher than in MIBK phase. When the HMF yields of MIBK and DES phases were combined, the highest HMF yield obtained in this study increased from 17.6 to 44.5%. It was from the reaction with CrCl3·6H2O added as the co-catalyst (110 °C, 20 min, 15 wt% water; Fig. 8d). Without the co-catalyst the highest total HMF yield was 37.3% (120 °C, 20 min, 15 wt% water; Fig. 8b). Based on the total HMF yields, it seems that Lewis acid co-catalyst might have had some effect on the conversion reaction, which would be in line with e.g., a previous study of Chen et al. (2020). Anyhow, the extraction process of HMF from DES during and after the conversion reaction needs to be studied further in future, so that HMF can be effectively removed from the DES.
Conclusions
Choline chloride:glycolic acid (1:3) DES was studied as a reaction medium for the synthesis of HMF from apple pomace. Glycolic acid component of DES acted as the Bronsted acid catalyst for the dehydration reaction of the sugar components of the apple pomace. MIBK was added to the reaction mixture to form a biphasic reaction system, so that HMF could be extracted from the DES already during the reaction. Based on the HPLC analysis of both phases of the reaction system, the extraction process was not sufficient. The DES phase contained a considerable amount of HMF, even though it was extracted with additional amounts of MIBK after the reaction. The reaction conditions had some effect on the HMF yield. The yield increased clearly when water was added to the reaction mixture. Some increase in the yield was also achieved by adding Lewis acid, CrCl3, as the co-catalyst to the reaction. Increasing reaction temperature, however, decreased the yield. Reaction time on the other hand did not seem to have much effect on the HMF yield. The highest total HMF yield (44.5%) was achieved at 110 °C in 10 min with 15 wt% H2O, and 0.01 g CrCl3 as co-catalyst. Without the co-catalyst, the highest achieved HMF yield was 37.3% (120 °C, 20 min, 15 m% H2O). Overall, the study showed that fruit processing industry waste stream, apple pomace, can be used as feedstock for HMF production. In addition, the results indicated that choline chloride:glycolic acid (1:3) DES is a potential reaction medium, as well as a Bronsted acid catalyst for the conversion reaction. More research is needed though in future to improve the extraction process of HMF from DES during and after the reaction. Also, the recycling of DES should be studied.
References
Abbott AP, Boothby D, Capper G, Davies DL, Rasheed RK (2004) Deep eutectic solvents formed between choline chloride and carboxylic acids: versatile alternatives to ionic liquids. J Am Chem Soc 126:9142–9147. https://doi.org/10.1021/ja048266j
Chen W-C, Lin Y-C, Chu I-M, Wang L-F, Tsai S-L, Wei Y-H (2020) Feasibility of enhancing production of 5-hydroxymethylfurfural using deep eutectic solvents as reaction media in a high-pressure reactor. Biochem Eng J 154:107440. https://doi.org/10.1016/j.bej.2019.107440
Chen L, Xiong Y, Qin H, Qi Z (2022) Advances of ionic liquids and deep eutectic solvents in green processes of biomass-derived 5-hydroxymethylfurfural. Chemsuschem 15:e202102635. https://doi.org/10.1002/cssc.202102635
Choudhary V, Pinar AB, Lobo RF, Vlachos DG, Sandler SI (2013) Comparison of homogeneous and heterogeneous catalysts for glucose to fructose isomerization in aqueous media. Chemsuschem 6:2369–2376. https://doi.org/10.1002/cssc.201300328
Dhillon GS, Kaur S, Brar SK (2013) Perspective of apple processing wastes as low-cost substrates for bioproduction of high value products: a review. Renew Sustain Energy Rev 27:789–805. https://doi.org/10.1016/j.rser.2013.06.046
Gassara F, Ajila CM, Brar SK, Tyagi RD, Verma M, Valero JR (2012) Lignin analysis using microwave digestion. Biotechnol Lett 34:1811–1815. https://doi.org/10.1007/s10529-012-0991-7
Hammond OS, Bowron DT, Edler KJ (2017) The effect of water upon deep eutectic solvent nanostructure: an unusual transition from ionic mixture to aqueous solution. Angew Chem Int Ed 56:9782–9785. https://doi.org/10.1002/anie.201702486
Hu S, Zhang Z, Song J, Zhou Y, Han B (2009) Efficient conversion of glucose into 5-hydroxymethylfurfural catalyzed by a common Lewis acid SnCl4 in an ionic liquid. Green Chem 11:1746–1749. https://doi.org/10.1039/B914601F
Istasse T, Richel A (2020) Mechanistic aspects of saccharide dehydration to furan derivatives for reaction media design. RSC Adv 10:23720–23742. https://doi.org/10.1039/D0RA03892J
Kennedy MD, Lu Y, Foo LY, Newman RH, Sims IM, Bain PJS, Hamilton B, Fenton G (1999). In: Linskens HF, Jackson JF (eds) Modern methods of plant analysis, vol 20. Springer, Heidelberg. https://doi.org/10.1007/978-3-662-03887-1_4
Massias A, Boisard S, Baccaunaud M, Leal Calderon F, Subra-Paternault P (2015) Recovery of phenolics from apple peels using CO2 + ethanol extraction: kinetics and antioxidant activity of extracts. J Supercrit Fluids 98:172–182. https://doi.org/10.1016/j.supflu.2014.12.007
Melikoğlu AY, Bilek SE, Cesur S (2019) Optimum alkaline treatment parameters for the extraction of cellulose and production of cellulose nanocrystals from apple pomace. Carbohydr Polym 215:330–337. https://doi.org/10.1016/j.carbpol.2019.03.103
Mika LT, Cséfalvay E, Németh A (2018) Catalytic conversion of carbohydrates to initial platform chemicals: chemistry and sustainability. Chem Rev 118:505–613. https://doi.org/10.1021/acs.chemrev.7b00395
Perussello CA, Zhang Z, Marzocchella A, Tiwari BK (2017) Valorization of apple pomace by extraction of valuable compounds. Compr Rev Food Sci Food Saf 16:776–796. https://doi.org/10.1111/1541-4337.12290
Qi X, Watanabe M, Aida TM, Smith RL (2012) Synergistic conversion of glucose into 5-hydroxymethylfurfural in ionic liquid-water mixtures. Bioresour Technol 109:224–228. https://doi.org/10.1016/j.biortech.2012.01.034
Rachana S, Gupta DK (2010) Utilization of pomace from apple processing industries: a review. J Food Sci Technol 47:365–371. https://doi.org/10.1007/s13197-010-0061-x
Rodriguez-Rodriguez N, van den Bruinhorst A, Kollau LJBM, Kroon MC, Binnemans K (2019) Degradation of deep-eutectic solvents based on choline chloride and carboxylic acids. ACS Sustain Chem Eng 7:11521–11528. https://doi.org/10.1021/acssuschemeng.9b01378
Rusanen A, Lappalainen K, Kärkkäinen J, Lassi U (2021) Furfural and 5-hydroxymethylfurfural production from sugar mixture using deep eutectic solvent/MIBK system. ChemistryOpen 10:1004–1012. https://doi.org/10.1002/open.202100163
Rusanen A, Lappalainen K, Kärkkäinen J, Tuuttila T, Mikola M, Lassi U (2019) Selective hemicellulose hydrolysis of Scots pine sawdust. Biomass Convers Biorefin 9:283–291. https://doi.org/10.1007/s13399-018-0357-z
Szymanska-Chargot M, Chylinska M, Kruk B, Zdunek A (2015) Combining FT-IR spectroscopy and multivariate analysis for qualitative and quantitative analysis of the cell wall composition changes during apples development. Carbohydr Polym 115:93–103. https://doi.org/10.1016/j.carbpol.2014.08.039
Tang X, Zuo M, Li Z, Liu H, Xiong C, Zeng X, Sun Y, Hu L, Liu S, Lei T, Lin L (2017) Green processing of lignocellulosic biomass and its derivatives in deep eutectic solvents. Chemsuschem 10:2696–2706. https://doi.org/10.1002/cssc.201700457
Teong SP, Yia G, Zhang Y (2014) Hydroxymethylfurfural production from bioresources: past, present and future. Green Chem 16:2015–2026. https://doi.org/10.1039/C3GC42018C
Tsilomelekis G, Orella MJ, Lin Z, Cheng Z, Zheng W, Nikolakis V, Vlachos DG (2016) Molecular structure, morphology and growth mechanisms and rates of 5-hydroxymethyl furfural (HMF) derived humins. Green Chem 18:1983–1993. https://doi.org/10.1039/C5GC01938A
Villas-Boas SG, Esposito E, Matos de Mendonc M (2004) Novel lignocellulolytic ability of Candida utilis during solid-substrate cultivation on apple pomace. World J Microbiol Biotechnol 18:541–545. https://doi.org/10.1023/A:1016350612380
Wilczyński K, Kobus Z, Dziki D (2019) Effect of press construction on yield and quality of apple juice. Sustainability 11:3630–3644. https://doi.org/10.3390/su11133630
Yu IKM, Tsang DCW (2017) Conversion of biomass to hydroxymethylfurfural: a review of catalytic systems and underlying mechanisms. Bioresour Technol 238:716–732. https://doi.org/10.1016/j.biortech.2017.04.026
Zhao Y, Lu K, Xu H, Zhu L, Wang S (2021) A critical review of recent advances in the production of furfural and 5-hydroxymethylfurfural from lignocellulosic biomass through homogeneous catalytic hydrothermal conversion. Renew Sustain Energy Rev 139:110706. https://doi.org/10.1016/j.rser.2021.110706
Zuo M, Jia W, Feng Y, Zeng X, Tang X, Sun Y, Lin L (2021a) Effective selectivity conversion of glucose to furan chemicals in the aqueous deep eutectic solvent. Renew Energy 164:23–33. https://doi.org/10.1016/j.renene.2020.09.019
Zuo M, Wang X, Wang Q, Zeng X, Zeng X, Lin L (2021b) Aqueous-natural deep eutectic solvent-enhanced 5-hydroxymethylfurfural production from glucose, starch, and food wastes. Chemsuschem 15:e202101889. https://doi.org/10.1002/cssc.202101889
Funding
Open Access funding provided by University of Oulu. This work was supported by Kone Foundation (Grant Number 201903073), Maj and Tor Nessling Foundation (Grant Number 201800070), and Oulun läänin talousseuran maataloussäätiö.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Lehtinen, J., Rusanen, A., Kärkkäinen, J. et al. Production of 5-hydroxymethylfurfural from apple pomace in deep eutectic solvent. Chem. Pap. 78, 173–180 (2024). https://doi.org/10.1007/s11696-023-03055-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11696-023-03055-0