Abstract
For quantitation of amlodipine (AML) and perindopril (PER) in their authentic, pharmaceutical formulations and spiked human plasma, a simple, sensitive, validated and inexpensive spectrofluorimetric method has been developed. The proposed method is developed to be based on quantitative quenching effect of two antihypertensive drugs on Eosin Y's native fluorescence which was achieved by developing binary complexes between each of the cited drugs in an acidic environment using acetate buffer (pH 4.4) with Eosin Y. Fluorescence quenching was recorded at 544 nm after excitation at 425 nm. For AML and PER, calibration curves were obtained over the range of 0.3–3.0 µg/mL and 0.2–2.0 µg/mL, respectively, with correlation coefficients of 0.9993 and 0.9995, respectively. The developed method was validated according to ICH guidelines. The proposed spectrofluorimetric method is regarded new and sensitive. As a result, the proposed method might be used to estimate the quality of the cited drugs in their pharmaceutical formulations and biological fluid.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
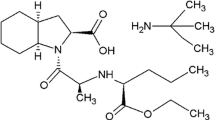
Amlodipine (AML), is a dihydropyridine compound with antihypertensive and antianginal effects with a nomenclature of 3-O-ethyl 5-O-methyl 2-(2-aminoethoxymethyl)-4-(2-chlorophenyl)-6-methyl-1,4-dihydropyridine-3,5-dicarboxylate, (Fig. 1-a). Various analytical techniques have been reported for determination of AML, either alone or in combination with other drugs, including, spectrophotometric techniques (Attimarad et al. 2019; Erden et al. 2016; Hegazy et al. 2015; Karadurmuş et al. 2018; Khachornsakkul and Dungchai 2020; Mahant et al. 2016; Saad et al. 2016; Siridevi et al. 2019), spectrofluorimetric techniques (Attala et al. 2020; Dinç et al. 2017; El-Kosasy et al. 2015; Ibrahim et al. 2015; Mabrouk et al. 2018; Mohamed et al. 2016; Mohammed 2015; Nasr and Shalan 2020; Sh Shalan et al. 2015; Shereen Shalan and Nasr 2019), electrochemical techniques (Erden et al. 2016; Wei et al. 2016) and chromatographic techniques such as TLC-densitometry (Patel et al. 2020a, b), LC–MS/MS (Rezk and Badr 2020) and HPLC (Alaama et al. 2015; Attimarad et al. 2020; Desai et al. 2019; Duraisamy and Jaganathan 2017; Ebeid et al. 2015; Karadurmuş et al. 2018; Mahant et al. 2016; Nagamani et al. 2020; Naik et al. 2020; Patel et al. Patel et al. 2020a, b; Saleh et al. 2020; Saputri et al. 2018). Perindopril (PER), is an antihypertensive non-sulfhydryl angiotensin converting enzyme (ACE) inhibitor used to treat hypertension and stable coronary artery disease with nomenclature of (2S,3aS,7aS)-1-[(2S)-2-[[(2S)-1-ethoxy-1-oxopentan-2-yl]amino]propanoyl]-2,3,3a,4,5,6,7,7a-octahydroindole-2-carboxylic acid (Fig. 1-b). For determination of PER alone or in combination, a variety of analytical techniques have been reported including spectrophotometric techniques (El-Bagary et al. 2016; Hegazy et al. 2015; Karadurmuş et al. 2018; Magdy et al. 2018; Rahman et al. 2017; Saad et al. 2016; Siridevi et al. 2019), spectrofluorimetric techniques (Al-Haj Sakur et al. 2015; Fael and Sakur 2015a, b; Sakur et al. 2015) and chromatographic techniques including TLC-densitometry (Patel et al. 2020a, b), LC–MS/MS (Rezk and Badr 2020) and HPLC (Duraisamy and Jaganathan 2017; Hassan et al. 2020; Hemdan et al. 2018; Karadurmuş et al. 2018; Naik et al. 2020; Patel et al. 2020a, b; Saleh et al. 2020).
Eosin Y is a sodium salt where the carboxyl and phenolic hydroxyl groups lost their protons and the resultant charge is neutralized by two sodium cations, with nomenclature of (2-(2,4,5,7-tetrabromo-6-oxido-3-oxo-3H-xanthen- 9-yl) benzoate) (Fig. 1-c). It has been frequently utilized as histological dye and was applied for estimation the concentration of certain amino compounds by forming ion pair complexes.
The spectrofluorimetric methods are simple, more sensitive than some other analytical methods compared for example with spectrophotometric methods, introduce a chance for estimation of different drugs in biological fluids in very small concentrations and more economic compared for example with chromatographic methods. On the other hand, the spectrofluorimetric methods are limited for certain types of drugs.
Until now, there is no spectrofluorimetric method for determination of the cited drugs via Eosin Y has been published, so in this work a simple, sensitive, validated and cheap spectrofluorimetric method was developed primarily in order to quantify AML and PER in their powders, pharmaceutical formulations and biological fluids.
Experimental
Apparatus
A Perkin Elmer LS-45 Luminescence spectrometer (UK) was used to get the required emission spectra. It has a xenon flash lamp and a quartz cell of 1 cm, and connected to an IBM PC. A Biochrom Ultrospec 700/700 PC, dual beam, ultraviolet (UV)–visible spectrometer (Cambridge, UK), with 1 cm quartz cells were accustomed. A centrifuge (Bramsen ECCO, Germany) and pH meter were used.
Materials
Chemicals and reagents
Without additional purification, all compounds and solvents are of analytical grade. Eosin Y was acquired from Sigma-Aldrich Chemie GmbH, (Steinheim, Germany) and was prepared in distilled water (5 × 10−4 M). Methanol, sodium acetate and acetic acid were obtained from El-Nasr Pharmaceutical Chemicals Co., (Abu-Zabaal, Cairo, Egypt). The acetate buffer is obtained by mixing 0.1 M acetic acid and 0.1 M sodium acetate then completed with distilled water to get pH range of 3.0 to 5.5. Amlodipine of 98.76% purity and Perindopril of 99.45% purity regarding to the reported methods (Basavaiah et al. 2006) and (Suresh 2012), respectively, were kindly provided by Eva Pharma Pharmaceutical Industries, (Giza, Egypt).
Pharmaceutical formulation
Alkapress® oral tablets (batch No: 302, manufactured by Hikma Pharma, Cairo, Egypt) which contain 5 mg of AML per one tablet. Protectopril® oral tablets (batch No: 200282, manufactured by Al Andalous for Pharmaceutical Industries, 6th October City, Egypt) which contain 8 mg of PER per one tablet.
Standard solutions
Ten mg of either AML or PER was accurately weighed, transferred to a volumetric flask of 100-mL and dissolved in 50 mL methanol by shaking. The volume was completed to the line with same solvent to get 0.1 mg/mL stock solution of cited drugs. To get a working solution of 10.0 μg/mL, 10 mL of AML and PER stock solutions (0.1 mg/mL) was accurately transferred into a volumetric flask of 100-mL, and volume was filled up with distilled water.
Procedure
General analytical procedure
AML and PER solutions were taken from their working solutions (10.0 μg/mL) to a set of arranged volumetric flasks of 10 mL at different concentrations levels from 0.3 to 3.0 μg /mL for AML and 0.2 to 2.0 μg /mL for PER. After that, 1.2 mL of acetate buffer solution (pH 4.4) and 1.0 mL of Eosin Y solution (5 × 10−4 M) were added. Then, the volume was completed with distilled water. After excitation at 425 nm, the fluorescence of the produced solutions was recorded at 544 nm. In their calibration curves, the decrease in fluorescence intensity for the produced solutions were recorded and plotted against both AML and PER concentrations.
Procedure for pharmaceutical formulations
Ten tablets of each Alkapress® tablets (AML) and Protectopril® tablets (PER) were finely ground and thoroughly combined. Amounts of accurately weighed powdered tablets, containing (10 mg) of both AML and PER were transferred to a volumetric flask of 100 mL. A volume of 50 mL methanol was added and sonication for 20 min was performed before cooling and completing the volume with same solvent. After that, filtration was performed to have a stock solution of 0.1 mg/mL of both AML and PER. Then, dilution with methanol was done to get 10.0 μg/mL for both AML and PER solutions. The steps described in the general analytical procedure were followed to estimate AML concentration in Alkapress® tablets and PER concentration in Protectopril® tablets using their corresponding regression equation.
Procedure for spiked human plasma samples
A volume of 5-mL drug free blood sample was drained from the arm of a healthy male volunteer of 35 years into a previously heparinized tube. The separation of needed plasma is done through centrifugation of the obtained blood sample for 20 min at 4000 rpm. Aliquot of 1.0 mL of the separated plasma was taken and 1.0 mL of either standard drug solution having concentration of (50, 100, 150 μg/mL) for AML and (50, 80, 100 μg/mL) for PER, were added, mixed well, stand for 10 min and 2.0 ml acetonitrile was added for deproteinization, then completed to the 10 mL with distilled water. The mixed components were centrifuged at 4000 rpm for 30 min. One mL of the clear supernatant was taken from these solutions and analyzed using the previously described spectrofluorimetric method.
Result and discussion
Eosin is a dye with acidic characters which is commonly used as an ion-pairing tool for the spectrofluorimetric determination of amino compounds via the production of binary complexes. The electrostatic interaction between the cited drugs cationic functional groups and the eosin anion formed at (pH 4.4) resulted in the complex formation. After excitation at 425 nm, the intensity of Eosin Y's fluorescence at 544 nm decreased upon complex formation (Fig. 2).
Experimental parameters optimization
All various parameters that influence the production and stability of the generated complex between the cited drugs and Eosin Y such as: buffer volume, buffer pH, and Eosin volume were studied and optimized.
Buffer pH and volume optimization
The medium had to be acidic to induce ion pair reaction between cited drugs and Eosin Y completely and hence, pH was regarded as an important variable. The pH of acetate buffer solutions of the range of 3.0 to 5.5, were examined but the pH of 4.4 was chosen since it produced the highest ΔRFI readings, (Fig. 3). The effect of various volumes of the acetate buffer (pH 4.4) were investigated (0.5 to 2.0 mL). The best result that showed the greatest ΔRFI values was attained when 1.2 mL of acetate buffer solution was used, (Fig. 4).
Eosin volume optimization
To achieve the highest sensitivity of the proposed method, several volumes of Eosin Y (5 × 10−4 M), (0.5–2.0 mL) were tested. The ΔRFI values showing the highest intensity when1.0 mL of Eosin Y solution was used, (Fig. 5).
Method validation
The described method was validated in accordance with three commendations of International Conference on Harmonization (ICH) (2005).
Linearity of calibration curve
Calibration curves of both AML and PER were built by plotting ΔRFI value versus their corresponding concentrations. Linearity of the described method was recorded in concentration area of 0.3–3.0 µg /mL and 0.2–2.0 µg/mL with 0.9993 and 0.9995 as correlation coefficient for AML and PER, respectively. The parameters of the regression equation that were obtained are shown in Table 1.
Limits of quantitation and detection
Values of 0.224 and 0.074 µg/mL were calculated as quantitation and detection limits for AML while the values for PER were 0.121 and 0.0399 µg/mL. The lowest values of these limits provide the developed method's highest sensitivity.
Precision
The developed method's precision was tested at inter-day and intra-day precision levels by estimating standard solution at three concentrations (0.5, 1.0, and 1.5 µg/mL) for AML and (0.5, 0.8, and 1.0 µg/mL) for PER. Each concentration was measured three times applying the general procedure, through three consecutive days for intra-day or in three different times within the day, for inter-day precision. The acceptable percentage recoveries, SD and RSD values were obtained and displayed in Table 2.
Robustness
Small variations in method characteristics have no effect on the created method's effectiveness. Buffer solution volume, pH, and Eosin volume were examined. Table 3 shows that the developed method was robust as minor variations in any of these factors had no discernible impact on the method's effectiveness.
Accuracy
The suggested method's accuracy was tested by evaluating three concentration levels within the chosen calibration ranges (0.5, 1.0, 1.5 µg/mL) for AML and (0.5, 0.8, 1.0 µg/mL) for PER. For each single concentration, the percentage recovery, SD and RSD were estimated and shown in Table 4. The proposed method was then used to estimate AML and PER in pharmaceutical formulations applying standard addition technique, that involved spiking each of Alkapress® oral tablet and Protectopril® oral tablet solutions with suitable amounts of AML and PER standard solutions and analyzing the solutions as previously mentioned. The concentrations of AML and PER were then calculated using their corresponding regression equations, and percentage recoveries, SD and RSD were calculated. The accuracy of the developed method was proved by the satisfactory results of the standard addition technique, also that results prove that there is no interference of the occurred additives with the drugs concentrations as shown in Table 5.
Applications to pharmaceutical formulations
Oral tablets of Alkapress® and Protectopril® were evaluated as described in the general procedure. Accebtable percentage recoveries and standard deviations of 98.96 ± 0.91 and 99.04 ± 0.23 were obtained, which statistically comparable to the values obtained using the reported methods (Basavaiah et al. 2006) and (Suresh 2012) for AML and PER, respectively, using the tabulated t-test and F- test at 95% confidence limit. Table 6 illustrates the acquired t-test and F- test values, which were detected to be lower than the tabulated ones, indicating that the developed method has no discernible change in precision and accuracy between the proposed and the reported methods.
Applications to spiked human plasma
As the proposed method is sensitive, so it was expected to give acceptable recovery in spiked human plasma. The developed method was applied for the analysis of the studied drugs in spiked human plasma by mixing certain volumes of plasma and standard drugs solutions with different concentration levels within the recommended range of AML and PER. The investigated concentration levels were (0.5–1.0–1.5 µg/mL) and (0.5–0.8–1.0 µg/mL) for AML and PER, respectively. Percentage recoveries, SD and RSD of each concentration level show suitability of this method for analysis of AML and PER in spiked human plasma as shown in Table 7.
Conclusion
The aim of that work is to introduce a new spectrofluorimetric method for quantitation of AML and PER in raw material, pharmaceutical formulations and spiking human plasma that is simple, precise, sensitive and time efficient. The developed method is a good technique for quality control of the cited drugs because of its sensitivity and simplicity. The developed method is a more sensitive spectrofluorimetric method than some other existing spectrofluorimetric methods that shown in Table 8.
Data availability
Data available on request.
References
Al-Haj Sakur A, Chalati T, Fael H (2015) Selective spectrofluorimetric method for the determination of perindopril erbumine in bulk and tablets through derivatization with o-phthalaldehyde in presence of 3-mercaptopropionic acid. Int J Acad Sci Res 3(2):26–37
Alaama M, Uddin AH, Mohamad HJ, Amiruddin NS, Abbas S (2015) Development and validation of reversed phase high performance liquid chromatographic method for determination of amlodipine. Trop J Pharm Res 14(4):663–669
Attala K, Eissa MS, Hasan MA, El-Henawee MM, Abd El-Hay SS (2020) An enhanced first derivative synchronous spectrofluorimetric method for determination of the newly co-formulated drugs, amlodipine and celecoxib in pharmaceutical preparation and human plasma. Spectrochim Acta A Mol Biomol Spectrosc 240:118533–118542
Attimarad M, Venugopala KN, Aldhubiab BE, Nair AB, Sreeharsha N, Pottathil S, Akrawi SH (2019) Development of UV spectrophotometric procedures for determination of amlodipine and celecoxib in formulation: use of scaling factor to improve the sensitivity. J Spectrosc 2019:1–10
Attimarad M, Venugopala KN, Sreeharsha N, Aldhubiab BE, Nair AB (2020) Validation of rapid RP-HPLC method for concurrent quantification of amlodipine and celecoxib in pure and formulation using an experimental design. Microchem J 152:104365
Basavaiah K, Chandrashekar U, Nagegowda P (2006) Titrimetric and modified spectrophotometric methods for the determination of amlodipine besylate using bromate-bromide mixture and two dyes. Sci Asia 32:271–278
Desai PR, Mehta PJ, Chokshi AB (2019) Stability indicating RP-HPLC method development and validation for simultaneous quantification of 15 organic impurities of olmesartan medoxomil, amlodipine and hydrochlorothiazide in combined dosage form. Chromatographia 82(5):819–833
Dinç E, Ertekin ZC, Büker E (2017) Multiway analysis methods applied to the fluorescence excitation-emission dataset for the simultaneous quantification of valsartan and amlodipine in tablets. Spectrochim Acta A Mol Biomol Spectrosc 184:255–283
Duraisamy K, Jaganathan K (2017) Method development and validation of HPLC tandem/mass spectrometry for quantification of perindopril arginine and amlodipine besylate combination in bulk and pharmaceutical formulations. Res Pharm Sci 12(4):307–314
Ebeid WM, Elkady EF, El-Zaher AA, El-Bagary RI, Patonay G (2015) Simultaneous determination of aliskiren hemifumarate, amlodipine besylate and hydrochlorothiazide in spiked human plasma using UPLC-MS/MS. J Chromatogr Sci 53(7):1178–1184
El-Bagary RI, Mowaka S, Elkady EF, Attallah MA (2016) Validated spectrophotometric methods for determination of weakly UV absorbed perindopril arginine in bulk and combined dosage form. Anal Chem Lett 6(6):766–782
El-Kosasy AM, Tawakkol SM, Ayad MF, Sheta AI (2015) New methods for amlodipine and valsartan native spectrofluorimetric determination, with factors optimization study. Talanta 143:402–413
Erden S, Bayraktepe DE, Yazan Z, Dinç E (2016) TiO 2 modified carbon paste sensor for voltammetric analysis and chemometric optimization approach of amlodipine in commercial formulation. Ionics 22(7):1231–1240
Fael H, Aa-H S (2015a) Novel Spectrofluorimetric method for the determination of perindopril erbumine based on charge transfer reaction with 7-Hydroxycoumarin. J Fluoresc 25(4):811–818
Fael H, Aa-H S (2015b) Novel Spectrofluorimetric method for the determination of perindopril erbumine based on fluorescence quenching of Rhodamine B. J Fluoresc 25(6):1577
Hassan SA, Nashat NW, Elghobashy MR, Abbas SS, Moustafa AA (2020) Stability-indicating RP-HPLC and CE methods for simultaneous determination of bisoprolol and perindopril in pharmaceutical formulation: a comparative study. J Chromatogr Sci 58(8):747–758
Hegazy MA, Abbas SS, Zaazaa HE, Essam HM (2015) Conventional univariate versus multivariate spectrophotometric assisted techniques for simultaneous determination of perindopril arginin and amlodipine besylate in presence of their degradation products. Spectrochim Acta A Mol Biomol Spectrosc 150:940–960
Hemdan A, Magdy R, Farouk M (2018) Response surface design as a powerful tool for the development of environmentally benign HPLC methods for the determination of two antihypertensive combinations: greenness assessment by two green analytical chemistry evaluation tools. J Sep Sci 41(16):3213–3223
Ibrahim F, El-Enany N, Shalan S, Abo Shabana R (2015) Second-derivative synchronous spectrofluorimetric determination of nebivolol hydrochloride and amlodipine besylate in their combined dosage form. Lumin 30(7):1011–1019
Ich. Q2 (R1) (2005) Validation of analytical procedures: Text and methodology, international conference on harmonization. Geneva
Karadurmuş L, Gümüştaş M, Kurbanoğlu S, Uslu B, Özkan SA (2018) A novel core-shell-based chromatographic method supported by ratio derivative spectrophotometry for the simultaneous determination of perindopril, indapamide, and amlodipine ternary mixtures. Turk J Chem 42(5):1408–1419
Khachornsakkul K, Dungchai W (2020) A portable reflective absorbance spectrophotometric smartphone device for rapid and highly accurate determination of amlodipine in pharmaceutical formulation and human urine sample. Anal Sci 20:349–375
Mabrouk MM, Hammad SF, El-Malla SF, Elshenawy EA (2018) Simultaneous determination of amlodipine and metoprolol in their combined dosage form using a synchronous fluorescence spectrofluorimetric method. Lumin 33(2):364–369
Magdy R, Hemdan A, Fares N, Farouk M (2018) Stepwise optimization strategies utilizing recent univariate and multivariate methods for determination of the partially overlapped mixture perindopril and indapamide. Anal Chem Lett 8(4):486–502
Mahant BD, Rajoriya V, Jain RK, Kashaw V (2016) UV-spectrophotometric and stability indicating RP-HPLC method for the simultaneous estimation of amlodipine besylate and indapamide. Anal Chem Lett 6(4):354–370
Mohamed A-MI, Omar MA, Hammad MA, Mohamed AA (2016) Development and validation of highly sensitive stability indicating spectrofluorimetric method for determination of amlodipine in pharmaceutical preparations and human plasma. J Fluoresc 26(6):2141–2149
Ta-E-F M (2015) Native and synchronous spectrofluorimetric methods for simultaneous determination of amlodipine besylate/valsartan combination in tablets. Asian J Sci Technol 6:1690–1698
Nagamani P, Manjunath S, Kumar TH (2020) Development and validation of RP-HPLC method for estimation of amlodipine besylate and celecoxib in pharmaceutical formulation. JDDT 10(6):31–36
Naik S, Nazareth C, Pereira S (2020) A novel HPLC cleaning validation and assay method for the simultaneous estimation of Perindopril and Amlodipine. Res J Pharma Tech 13(12):5919–5923
Nasr JJ, Shalan S (2020) Simultaneous estimation of amlodipine and atorvastatin by micelle-augmented first derivative synchronous spectrofluorimetry and multivariate analysis. Spectrochim Acta A Mol Biomol Spectrosc 224:117430–117438
Patel K, Chhalotiya U, Kachhiya H, Patel J, Shah D, Nagda D (2020a) Simultaneous quantification of perindopril erbumine, indapamide and amlodipine besylate in newer combination of anti-hypertensive drugs in pharmaceutical dosage form by thin layer chromatography method. Sep Sci Plus 3(5):175–184
Patel KP, Chhalotiya UK, Kachhiya HM, Patel JK (2020b) A new RP–HPLC method for simultaneous quantification of perindopril erbumine, indapamide, and amlodipine besylate in bulk and pharmaceutical dosage form. Future J Pharm Sci 6(1):1–9
Rahman N, Rahman H, Haque SM (2017) Kinetic spectrophotometric method for the determination of perindopril erbumine in pure and commercial dosage forms. Arab J Chem 10:831–838
Rezk MR, Badr KA (2020) Determination of amlodipine, indapamide and perindopril in human plasma by a novel LC–MS/MS method: application to a bioequivalence study. Biomed Chromatogr 35:e5048–e5058
Saad AS, Abo-Talib NF, El-Ghobashy MR (2016) Novel ratio difference at coabsorptive point spectrophotometric method for determination of components with wide variation in their absorptivities. Spectrochim Acta A Mol Biomol Spectrosc 152:480–501
Sakur AA, Chalati T, Fael H (2015) Selective spectrofluorimetric method for the determination of perindopril erbumine in bulk and tablets through derivatization with dansyl chloride. J Anal Sci Tech 6(1):1–8
Saleh SS, Lotfy HM, Tiris G, Erk N, Rostom Y (2020) Analytical tools for greenness assessment of chromatographic approaches: application to pharmaceutical combinations of Indapamide. Perindopril Amlodipine Microchem J 159:105557
Saputri FA, Alawiyah A, Firsty AB, Megantara S, Kusuma ASW, Rusdiana T, Hasanah AN, Mutakin M, Surono IS, Abdulah R (2018) Development and validation of simple simultaneous analysis for Amlodipine and Glibenclamide by nonderivatization high-performance liquid chromatography-fluorescence. J Adv Pharm Technol Res 9(4):124–129
Shalan S, El-Enany N, Belal F (2015) Simultaneous determination of amlodipine besylate and valsartan using a micelle-enhanced first derivative synchronous spectrofluorimetric method and application in their co-formulated tablets. Anal Methods 7(19):8060–8068
Shalan S, Nasr JJ (2019) Simultaneous evaluation of losartan and amlodipine besylate using second-derivative synchronous spectrofluorimetric technique and liquid chromatography with time-programmed fluorimetric detection. R Soc Open Sci 6(4):190310–190323
Siridevi MP, Hemant KT, Srinivasa RY, Varaprasad RK (2019) Simultaneous spectrophotometric estimation of amlodipine besylate and Perindopril Erbumine in tablet formulation. Res J Pharm Tech 12(12):6101–6106
Sunitha G, Gayatri T, Manaswini Y, Mounika G, Anumolu P (2016) Synchronise derivative spectroflourimetric simultaneous quantification of nebivolol hydrochloride and amlodipine besylate. Pharm Lett 8(2):447–451
Suresh C (2012) Development of new spectrophotometric methods for the determination of perindopril in pharmaceutical dosage forms. IJNTPS 2(1):32–34
Wei Y, Wang H, Sun S, Tang L, Cao Y, Deng B (2016) An ultrasensitive electrochemiluminescence sensor based on reduced graphene oxide-copper sulfide composite coupled with capillary electrophoresis for determination of amlodipine besylate in mice plasma. Biosens Bioelectron 86:714–735
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB). No funding was received for this work.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Data collection and analysis were performed by all authors. The first draft of the manuscript was written by HS and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing financial interests.
Ethical approval
Not Applicable.
Consent to participate
Not applicable.
Consent for publication
Not Applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Salem, H., Abdelaziz, A., Derayea, S. et al. Spectrofluorimetric assay of amlodipine and perindopril in their raw materials, pharmaceutical formulations and spiked human plasma through the formation of complexes with Eosin Y. Chem. Pap. 77, 2817–2825 (2023). https://doi.org/10.1007/s11696-023-02668-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11696-023-02668-9