Abstract
Iron plays a significant role in the metabolism of cancer cells. In comparison with normal cells, neoplastic ones exhibit enhanced vulnerability to iron. Ferric ions target tumor via the ferroptotic death pathway—a process involving the iron-mediated lipid oxidation. Ferric ion occurs in complex forms in the physiological conditions. Apart from iron, ligands are the other factors to affect the biological activity of the iron complexes. In recent decades the role of iron chelates in targeting the growth of the tumor was extensively examined. The ligand may possess a standalone activity to restrict cancer’s growth. However, a wrong choice of the ligand might lead to the enhanced cancer cell’s growth in in vitro studies. The paper aims to review the role of iron complex compounds in the anticancer therapy both in the experimental and clinical applications. The anticancer properties of the iron complex rely both on the stability constant of the complex and the ligand composition. When the stability constant is high, the properties of the drug are unique. However, when the stability constant remains low, both components—ferric ions and ligands, act separately on the cells. In the paper we show how the difference in complex stability implies the action of ligand and ferric ions in the cancer cell. Iron complexation strategy is an interesting attempt to transport the anticancer Fe2+/3+ ions throughout the cell membrane and release it when the pH of the microenvironment changes. Last part of the paper summarizes the results of clinical trials and in vitro studies of novel iron chelates such as: PRLX 93,936, Ferumoxytol, Talactoferrin, DPC, Triapine, VLX600, Tachypyridine, Ciclopiroxamine, Thiosemicarbazone, Deferoxamine and Deferasirox.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Anticancer therapy aims to induce apoptosis in cancer cells, simultaneously leaving healthy ones unaffected. However, common defects in the executioner mechanism of cell death lead to the resistance to the therapy and make treatment process very elaborate and sometimes unpredictable. Nowadays, researchers aim to enhance the cytotoxic activity of the common chemotherapeutics by combining them with other anticancer compounds (Qi et al. 2017).
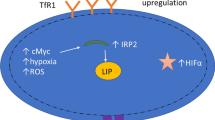
Unimpeded growth of cancer is promoted by various factors—including iron accessibility. Thus, neoplastic cells exhibit the iron-seeking phenotype by substantially increasing the demand for the microelement in comparison with healthy cells (Richardson et al. 2009). The phenomenon is induced by the dysregulation of the proteins related to iron metabolism. Iron cellular hemostasis is partially mediated by tumor suppressor genes and oncogenes (Manz et al. 2016). The hallmark makes cancer cells vulnerable to ferroptosis—a non-apoptotic nor necrotic type of cellular death (Tang and Kroemer 2020). The process includes lipid peroxidation as the result of Fenton’s reaction (see Fig. 1). Therefore, high concentration of iron (II) ions in the cytoplasm of the cells enhances the process (Xie et al. 2016). Nowadays, ferroptosis is examined as the potential tool in the anticancer therapy. Even though the process has already been extensively characterized, it remains not fully clear. One of the confusing points is the role of frataxin in the process (Du et al. 2020). Inside cells, frataxin acts as an iron buffer, protecting them from iron overload and also supporting the cells’ energy metabolism by enhancing the assembly of proteins which contain Fe-S clusters. The decrease in frataxin’s expression enhances ferroptosis (Du et al. 2020).
Quantity and the site of iron ions’ administration turn out to be crucial when it comes to its influence on carcinogenesis. On the one hand, the excess of iron leads to increased cancer risk, cancer initiation and tumor growth (Knekt et al. 1994). Conversely, the depletion of the microelement could be effectively used in treatment (Torti et al. 2018).
Ferric chelates are used as oral iron supplements as well as phosphate binders. The potential of iron (III) citrate and iron (III) EDTA complex in chemotherapy is currently being examined and yet show several ambiguous results. On the one hand, the iron (III)-EDTA decreases the expression of frataxin in colon adenocarcinoma, which leads to the induction of ferroptosis (Schulz et al. 2006). On the other hand, iron (III) citrate induces the progression of cancer, therefore cannot be considered as a potential therapeutic option (Scheers et al. 2018). Relatively low stability of iron (III) citrate leads to the delivery of citrate anions to cancer cells, which increases the Krebs cycle rate and therefore promotes progression (Scheers et al. 2018). Understanding the role of iron in the biology of cancer cells and the pathways of its significant influence might be crucial for the future examinations on therapy prospects. However, it is not yet well understood why some forms of iron mobilize and accelerate the cancer cells (Buss et al. 2005a; b).
The paper aims to overview the potential of iron complex compounds in the anticancer therapy. In the beginning we explain the mechanisms in which ferric ions induce apoptosis in cancer cells. Then we present the latest laboratory studies concerning the anticancer properties of iron chelates. Next, we move to the effects of the currently used diet supplements on the carcinogenesis process and explain the role of iron chelates stability on the safety of the medication. In the end we move to the clinical trials involving the use of iron compounds.
For the review paper, we have searched Google Scholar for most recent papers about the application of iron compounds in the anticancer therapy. We used only peer-reviewed papers from journals in the fields of medicine, biology or chemistry. Due to the focus of the paper on the potential of iron chelates in the anticancer therapy, we mostly omitted papers regarding the free iron’s role. Also, our search of papers included only the well-studied compounds. As for the calculations of concentrations of the ligand and various iron forms, we omitted the ionic strength and worked simply on molar concentrations.
Iron-mediated cytotoxicity against cancer
Markedly elevated concentration of iron increases overall death risk, including the elevated risk of cancer. Iron is responsible for catalyzing ROS production. Furthermore, it provides survival and progression of cells, which may have progressed to malignancy. Iron redox cycle is associated with reactive oxygen species (ROS) production. Hydroxyl radical is one of Fenton’s reaction product (see below).
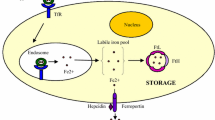
“Free” iron—non-transferrin plasma iron, consists of both Fe3+ and Fe2+. Iron on the III oxidative state is more stable in physiological conditions than iron on the second oxidative state (II). In neutral pH most Fe3+ precipitates as hydroxides. Some of Fe3+ chelates with low molecular weight chemical structures (ATP, ATP and GTP) stay as “free” iron in neutral pH. The fewer ligands are involved in chelation, the higher is the catalyzing activity of ferric ions to produce OH· radicals. Reducing agents (e.g., ascorbate, superoxide dismutase, glutathione) protect cells from the influence of ROS and Fenton’s reaction (Toyokuni 1996).
Peroxidative damage, spread by ROS, includes the injury of phospholipids from organelle’s membranes. Iron induces membrane disruption and distortion that alters the activity of the cell. These effects lead to the destruction of organelles such as mitochondria, microsomes or lysosomes. Iron is involved in the loss of cellular viability, by increasing phospholipase A (PLA) and lysophosphatidic activity (Bacon and Britton 1990). Interestingly, iron-citrate complex efficiently induces DNA strand breaks (Toyokuni and Sagripanti 1993).
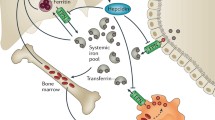
Tumoricidal action of macrophages is essential in inhibiting malignancy progression. Therefore sadly, iron dextran, iron salts, carbonyl iron and iron ferritin were reported to suppress anticancer activity of macrophages (Green et al. 1988). Interestingly, tumor cells express a large number of transferrin receptors.
Iron complexes in the anticancer therapy under in vitro and in vivo evaluation
Initially, iron chelators were developed to treat iron overload, now these agents are being repurposed to treat cancers. The agents have not yet been classified as anticancer drugs. There could be distinguished two main strategies of iron chelates’ action. The first one includes depletion of iron from cancer cells by the inhibition of cellular iron uptake or the promotion of iron metabolism. The second option focuses on the application of iron chelates to facilitate the redox cycling of iron, to generate cytotoxic reactive oxygen species (ROS) within tumor (Torti and Torti 2013). The other studies concern the influence of iron chelators on the inhibition of ribonucleotide reductase, which limits the iron-dependent enzymes for DNA synthesis or on excitation of cell cycle arrest (Lane et al. 2014). Studies concerning ferric chelates include Deferasirox (DFX), Deferoxamine (DFO), Thiosemicarbazone, Ciclopiroxamine (CPX), Tachypyridine, VLX600, citrate and EDTA.
Deferoxamine (DFO) and Deferasirox (DFX) display significant antiproliferative activity in human gastric cell lines. It is explained by the induction of G1 phase arrest and apoptosis. Apoptosis is induced by increased reactive oxygen species (ROS) production and c-Jun N-terminal kinase (JKN) activation (Kim et al. 2016).
In vitro and in vivo studies proved low toxicity of iron chelators and high efficiency in inhibiting tumor cell proliferation (Kicic et al. 2001). DFO, which showed promising antiproliferative properties on cancer cell lines, also demonstrated in vivo a dose-dependent antileukemic effect through the action on proliferation and differentiation of blasts (Estrov et al. 1987; Dezza et al. 1989). Furthermore, DFO promoted apoptosis of cancer cells in mammary adenocarcinoma-bearing rats. A low iron diet led to similar results indicating iron depletion as the mechanism of antitumor action of DFO (Wang et al. 1999; Jiang et al. 2002).
In the study by Saeki, DFX was proven to inhibit the proliferation of three hepatoma cell lines (HepG2, Hep3B and Huh7) in a dose-dependent manner (Saeki et al. 2016). Moreover, it induced apoptosis, by increasing caspase-3 activity (Saeki et al. 2016). DFX up-regulates mRNA expression of hepcidin, transferrin receptor 1 and HIF-1α levels. This leads to the inhibition of tumor growth via hypoxia-associated factors. Although DFX inhibited the proliferation of hepatoma cell lines and induced the activation of caspase-3 in vitro, the results were not supported by clinical studies.
In vitro antiproliferative activity of Thiosemicarbazone demonstrated an anticancer activity by increasing the expression of the growth and metastasis suppressor N-myc downstream-regulated gene 1 and its phosphorylation at Ser330 and Thr346. Furthermore, the agent augmented the expression of the cyclin-dependent kinase inhibitor p21, whilst decreasing cyclin D1 in pancreatic cells (Kovacevic et al. 2011). Several studies demonstrated potent inhibition of ribonucleotide reductase (RNR), which led to the reduced DNA replication and repair as an answer to Thiosemicarbazone activity (Finch et al. 2000).
Ciclopiroxolamine (CPX), a fungicidal with anticancer activity, is mediated through iron chelation and subsequent inhibition of iron-dependent enzymes. CPX downregulated DJ-1 (endogenous antioxidant oncogene), leading to ROS accumulation, impairing mitochondrial function and inducing apoptosis. Although in vitro studies were promising, the clinical use was abandoned because of the poor solubility of the drug, as well as its rapid metabolism into inactive glucuronide and quick clearance from the body (Weir et al. 2019).
A novel molecule VLX600 similar to CPX and DFO has been also classified as an iron chelator. VLX600 exhibits anticancer activity—in vitro by inhibiting RNA reductase of proliferating cells, decrease mitochondrial oxidative phosphorylation and eventually leads to decreased production of mitochondrial energy. Furthermore, VLX600 has proven to be more firm than other iron chelators in antiproliferative activity on colon cancer cells line.
Tachypyridine induces cell cycle arrest in the G2 phase in the HeLa cell line (cervical cancer) and CRC cell line (colorectal cancer). Interestingly, a non-iron analogue did not exhibit the same activity, suggesting the iron-related therapeutic activity (Brown et al. 2020).
Effects of iron complexes used as diet supplements on cancer
Currently, two iron-containing diet supplements are used by the patients. Ferric citrate and ferric-EDTA aim to increase total iron in patients’ organism; however, treatment with the drugs was reported to induce wide biological effects. Various forms of iron chelators impact tumorigenesis differently. Ferric citrate and ferric EDTA were discovered to promote colon cancer in mice. Further in vitro research proved that cells incubated with high concentration (0.5–2 mM) of ferric citrate induced amphiregulin (oncogenic growth factor) and its receptor EGFR (epithelial growth factor receptor) production and the activation of mitogen-activated protein kinase (MAPK), which lead to an increased risk of cancer progression. The dose of 0.5 mM ferric EDTA triggered the same up-regulation of amphiregulin, while other iron chelators such as ferrous sulfate did not evoke any. Furthermore, the smaller dose (0.05 mM) of both ferric citrate and ferric EDTA did not target amphiregulin (Scheers et al. 2018). Amphiregulin, as a growth factor, induces proliferation and inhibit apoptosis. Abnormal activity stimulated by iron chelators of amphiregulin leads to epithelial cancer development (Kariagina et al. 2010).
The study performed by Poljak-Blazi compared the influence of ferric-sorbitol-citric complex on the ROS production and proliferation in cervical carcinoma cell lines (HeLa, SiHa) and human papillomavirus (HPV) cell line (Poljak-Blazi et al. 2011). The cells were treated with Fe (III) ions at a concentration between 0.001 and 1 mM for various periods of time. As it was expected—some of the measurements exhibited positive outcome, it was also proven that Fe (III) ion treatment enhances survival of HPV 16 positive cells and may improve HPV oncogenesis.
Loreto et al. proved the antitumor activity of standalone EDTA on cancer cells of various lines. Efficacy varied between cell lines. Moreover, the toxicity of EDTA was higher in melanoma cells compared to normal melanocytes. The observation confirms that cancer cells are characterized by higher iron demand and therefore remain more sensitive to the environment full of iron chelators (Feril Jr et al. 2017). Despite the proven effectiveness of EDTA on cell lines, the compound showed no antitumor efficiency in tumor-bearing mice (El-Naggar and El-Said 2020). Additionally, interesting results were obtained with the use of ferric-EDTA complex. In vivo studies on mice showed that increased dietary iron intake (in the form of EDTA-iron complex) increased the risk of colitis-associated colorectal cancer development. In the group of mice supplemented with a double dose of iron, on day 17, 15 out of 16 mice had histopathological confirmation of the tumor. In contrast, in the normal diet control group, colorectal tumors were found in 6 mice (Seril et al. 2002). Interestingly, mice with DSS-inducted colitis with intraperitoneal administration of iron did not show an enhanced risk of colon carcinogenesis compared to the control group. The results were contrary in the group of mice treated with oral iron supplementation (iron-EDTA) in a double dose compared to the standard diet. In the group tumor incidence was significantly increased. Besides, enhanced expression of iNOS and COX-2 proteins has been observed (Seril et al. 2005).
The differences between two supplements might arise from the differences in the composition of their water solutions. To show the concentration of biologically active free ferric ions and stable complex compounds, calculations were performed. Data used to describe the solutions with two analyzed compounds are described in Table 1.
Standard in vitro experiments happen under pH = 7.4 and 25 °C temperature. However, the oral administration of the ferric citrate and ferric-EDTA implies the contact of the complex compounds with the acidic environment of the stomach (pH ~ 3) and duodenum (pH ~ 6). The stability constants (Table 1) are not affected by pH, but the free Fe3+ hydrolyses, thus decreasing the form of complexed iron in the solution (Atkins and De Paula 2010). Total concentration of iron can be described by the molar fraction formulas:
Due to the fact that ligand-to-metal ratio remains 1:1 in the analyzed compounds, the above formulas are equal to each other:
Combining the formulas with the equations for stability and hydrolysis constants:
Combining all together, the formula for the non-complexed iron concentration was derived:
where k for EDTA complex:
And k for citrate complex:
The data obtained by solving the equations were plotted to analyze the general distribution of iron forms in the working solutions under different pH conditions (see Fig. 1).
Due to the differences in the stability constants, solutions of both analyzed compounds differ in the fraction of complexed and non-complexed iron forms (see Fig. 2). Iron (III) citrate solution is composed of non-complexed iron and free citrate ions forms (Table 2). Conversely, in the iron (III)-EDTA solution almost all iron (III) ions are complexed with EDTA. Therefore, both components of iron citrate remain separated to some extent, whereas the EDTA complex in the same physiological pH remains mostly stable and does not dissociate. With the increase in H+ concentration, the dissociation of both complexes occurs; however in case of ferric-EDTA complex, the process does not provide as much free iron ions as the dissociation of less stable ferric citrate.
Presented data may be interesting in regard to novel anticancer therapy approaches toward cancer treatment with the use of iron complexes. By applying an unstable complex to the biological system, the compound acts as both of the separated compounds. However, if especially stable complexes are added to the cells or the organism, iron would not be accessible. Here the outcome of the therapy would probably be less beneficial and more restricted toward the exact EDTA-iron complex.
Clinical trials involving the iron complexes in oncology
In clinical settings, we can distinguish two approaches that evaluate the potential of iron to target cancer. Namely, iron depletion affects iron-dependent cellular processes, delivery of iron to trigger oxidative stress or induce ferroptosis and consequently eliminate tumor cells (Torti and Torti 2020). In clinical settings, both approaches are applied to enhance the sensitivity of cancer to chemotherapy and radiotherapy.
Among iron chelators, numerous clinical trials investigate Triapine as a potential drug against prostate cancer (NCT00054015), lung cancer (NCT00064064), breast cancer (NCT00095888) and pancreatic cancer (NCT00064051). The therapeutic potential of another iron chelator DpC (Dp4cycH4mT) is evaluated for advanced solid tumors (NCT02688101) (Guo et al. 2016).
Furthermore, gallium can be applied as an iron mimetic that dysregulates the iron-dependent proliferation of tumor cells (Chitambar 2012). Gallium nitrate underwent the clinical trial for brain tumors, neuroblastoma, rhabdomyosarcoma, non-Hodgkin's lymphoma, or refractory solid tumors (NCT00002543).
Studies show that lactoferrin the iron-binding glycoprotein has the potential to affect tumor iron homeostasis (Cutone et al. 2020). The recombinant human lactoferrin–talactoferrin entered clinical trials for renal cell carcinoma (NCT00095186) and lung cancer (NCT00923741) treatment.
The rationale of iron-based drugs application triggers the iron-based oxidative stress to eliminate tumor cells. Trujillo-Alonso et al. evaluated the application feasibility of ferumoxytol (Feraheme), a clinically approved iron oxide nanoparticle used to treat iron deficiency, in AML treatment (Trujillo-alonso et al. 2019).
Interestingly popular ascorbate shows an anticancer effect based on iron-dependent oxidative stress (Cancer et al. 2017). Multiple trials evaluate its efficiency among others for treatment of lung cancer (NCT02420314), pancreatic cancer (NCT02905578), prostate cancer (NCT01080352), bladder cancer (NCT04046094), sarcoma (NCT04634227) and glioblastoma (NCT02344355).
Also, the anticancer effect of ferroptosis-inducing agents is based on triggering the excess iron in cancer therapy (Su et al. 2020). The studies on Erastin suggests the effectiveness of ferroptosis inducers as anticancer drugs (Zhang et al. 2020). One clinical trial investigates Erastin’s analogue PRLX 93,936 for relapsed/refractory multiple myeloma (NCT01695590).
Table 2 summarizes the cancer response toward treatment with various iron complex compounds from both in vitro and in vivo studies.
Conclusions
Nowadays, anticancer research focuses on the development of novel and cytotoxic compounds. Ferric ions seem potent as the ferroptosis-inducing agents; thus, various studies concern the application of iron complex compounds in the anticancer therapy.
Iron-containing compounds, which are stable in the physiological conditions, act on cancer in a unique way. Namely, both components of the complex form an agent that interferes the metabolic pathways of the cells. Conversely, when the iron compound is not stable, the biological outcome depends on the interaction between both components of the complex. When the ligand poses a standalone cytotoxic effect on the cancer, the combination with ferric ions would be therapeutically beneficial. However, when the ligand stimulates the development and replication of the cell, the combination with ferric ions could even promote the progression of cancer.
Concluding, to obtain a good therapeutical potential of iron-containing drugs, the active compound should be characterized by (1) high stability of the complex and good standalone cytotoxicity or (2) low stability of the complex, but high cytotoxicity induced by the ligand that with the antiproliferative efficacy of ferric ions, would result in overall beneficial effect.
References
Atkins PW, De Paula J (2010) Atkins’ physical chemistry. Oxford University Press
Bacon BR, Britton RS (1990) The pathology of hepatic iron overload: a free radical-Mediated Process? Hepatology 11:127–137. https://doi.org/10.1002/hep.1840110122
Bedford MR, Ford SJ, Horniblow RD et al (2013) Iron chelation in the treatment of cancer: a new role for deferasirox? J Clin Pharmacol 53:885–891
Brown RAM, Richardson KL, Kabir TD et al (2020) Altered iron metabolism and impact in cancer biology, metastasis, and immunology. Front Oncol 10:476
Buss J, Torti F, Torti S (2005a) The role of iron chelation in cancer therapy. Curr Med Chem 10:1021–1034. https://doi.org/10.2174/0929867033457638
Buss JL, Greene BT, Turner JoLyn et al (2005b) Iron chelators in cancer chemotherapy. Curr Top Med Chem 4:1623–1635. https://doi.org/10.2174/1568026043387269
Cancer GBM, Ascorbate P, Schoenfeld JD et al (2017) Causes the differential susceptibility of NSCLC and O 2, – and H 2 O 2 -mediated disruption of fe metabolism causes the differential susceptibility of NSCLC and GBM cancer cells to pharmacological ascorbate. Cancer Cell 31:487-500.e8. https://doi.org/10.1016/j.ccell.2017.02.018
Chitambar CR (2012) Gallium-containing anticancer compounds. Future Med Chem 4:1257–1272
Cutone A, Rosa L, Ianiro G et al (2020) Lactoferrin’s anti-cancer properties: safety, selectivity, and wide range of action. Biomolecules 10:1–26
de Siqueira LRP, de Moraes Gomes PAT, de Lima Ferreira LP et al (2019) Multi-target compounds acting in cancer progression: focus on thiosemicarbazone, thiazole and thiazolidinone analogues. Eur J Med Chem 170:237–260
Dezza L, Cazzola M, Danova M et al (1989) Effects of desferrioxamine on normal and leukemic human hematopoietic cell growth: in vitro and in vivo studies. Leukemia 3:104–107
Du J, Zhou Y, Li Y et al (2020) Identification of Frataxin as a regulator of ferroptosis. Redox Biol 32:101483. https://doi.org/10.1016/j.redox.2020.101483
El-Naggar SA, El-Said KS (2020) Antitumor efficacy of edta co-treatment with cisplatin in tumor-bearing mice. Brazilian J Pharm Sci 56:1–8. https://doi.org/10.1590/s2175-97902019000418536
Enyedy ÉA, Nagy NV, Zsigó É et al (2010) Comparative solution equilibrium study of the interactions of copper (II), iron(II) and zinc(II) with triapine (3-Aminopyridine-2-carbaldehyde thiosemicarbazone) and related ligands. Eur J Inorg Chem 2010:1717–1728. https://doi.org/10.1002/ejic.200901174
Estrov Z, Tawa A, Wang XH et al (1987) In vitro and in vivo effects of deferoxamine in neonatal acute leukemia. Blood 69:757–761. https://doi.org/10.1182/blood.v69.3.757.757
Feril LB Jr, Ogawa K, Watanabe A et al (2017) Anticancer potential of EDTA: a preliminary in vitro study. Mathews J Cancer Sci 2:1–3
Finch RA, Liu MC, Grill SP et al (2000) Triapine (3-aminopyridine-2-carboxaldehyde-thiosemicarbazone): a potent inhibitor of ribonucleotide reductase activity with broad spectrum antitumor activity. Biochem Pharmacol 59:983–991. https://doi.org/10.1016/S0006-2952(99)00419-0
Gomathi H (2000) Chemistry and electrochemistry of iron complexes. Bull Electrochem 16:459–465
Green R, Esparza I, Schreiber R (1988) Iron inhibits the nonspecific tumoricidal activity of macrophages: a possible contributory mechanism for neoplasia in hemochromatosis. Ann N Y Acad Sci 526:301–309. https://doi.org/10.1111/j.1749-6632.1988.tb55514.x
Guo Z, Richardson DR, Kalinowski DS et al (2016) The novel thiosemicarbazone, di-2- pyridylketone 4-cyclohexyl-4-methyl-3- thiosemicarbazone ( DpC ), inhibits neuroblastoma growth in vitro and in vivo via multiple mechanisms. J Hematol Oncol 9:1–16. https://doi.org/10.1186/s13045-016-0330-x
Halabe Bucay A (2007) The biological significance of cancer: Mitochondria as a cause of cancer and the inhibition of glycolysis with citrate as a cancer treatment. Med Hypotheses 69:826–828. https://doi.org/10.1016/j.mehy.2007.02.002
Hayes TG, Falchook GF, Varadhachary GR et al (2006) Phase I trial of oral talactoferrin alfa in refractory solid tumors. In: Investigational new drugs. Springer, pp 233–240
Jiang XP, Wang F, Yang DC et al (2002) Induction of apoptosis by iron depletion in the human breast cancer MCF-7 cell line and the 13762NF rat mammary adenocarcinoma in vivo. Anticancer Res 22:2685–2692
Kantar C, Gillow JB, Harper-Arabie R et al (2005) Determination of stability constants of U(VI)-Fe(III)-citrate complexes. Environ Sci Technol 39:2161–2168. https://doi.org/10.1021/es048852c
Kariagina A, Xie J, Leipprandt JR, Haslam SZ (2010) Amphiregulin mediates estrogen, progesterone, and EGFR signaling in the normal rat mammary gland and in hormone-dependent rat mammary cancers. Horm Cancer 1:229–244. https://doi.org/10.1007/s12672-010-0048-0
Kicic A, Chua ACG, Baker E (2001) Effect of iron chelators on proliferation and iron uptake in hepatoma cells. Cancer 92:3093–3110. https://doi.org/10.1002/1097-0142(20011215)92:12%3c3093::AID-CNCR10107%3e3.0.CO;2-B
Kim JL, Lee DH, Na YJ et al (2016) Iron chelator-induced apoptosis via the ER stress pathway in gastric cancer cells. Tumor Biol 37:9709–9719. https://doi.org/10.1007/s13277-016-4878-4
Knekt P, Reunanen A, Takkunen H et al (1994) Body iron stores and risk of cancer. Int J Cancer 56:379–382. https://doi.org/10.1002/ijc.2910560315
Königsberger LC, Königsberger E, May PM, Hefter GT (2000) Complexation of iron(III) and iron(II) by citrate. Implications for iron speciation in blood plasma. J Inorg Biochem 78:175–184. https://doi.org/10.1016/s0162-0134(99)00222-6
Kovacevic Z, Chikhani S, Lovejoy DB, Richardson DR (2011) Novel thiosemicarbazone iron chelators induce up-regulation and phosphorylation of the metastasis suppressor N-myc down-stream regulated gene 1: a new strategy for the treatment of pancreatic cancer. Mol Pharmacol 80:598–609. https://doi.org/10.1124/mol.111.073627
Lane DJR, Mills TM, Shafie NH et al (2014) Expanding horizons in iron chelation and the treatment of cancer: Role of iron in the regulation of ER stress and the epithelial-mesenchymal transition. Biochim Biophys Acta - Rev Cancer 1845:166–181
Lui GYL, Obeidy P, Ford SJ et al (2013) The iron chelator, deferasirox, as a novel strategy for cancer treatment: Oral activity against human lung tumor xenografts and molecular mechanism of actions. Mol Pharmacol 83:179–190. https://doi.org/10.1124/mol.112.081893
Madan RA, Tsang K, Bilusic M et al (2013) Effect of Talactoferrin Alfa on the immune system in adults with non-small cell lung cancer. Oncologist 18:821–822. https://doi.org/10.1634/theoncologist.2013-0199
Manz DH, Blanchette NL, Paul BT et al (2016) Iron and cancer: recent insights. Ann N Y Acad Sci 1368:149–161. https://doi.org/10.1111/nyas.13008
Maqbool SN, Lim SC, Park KC et al (2020) Overcoming tamoxifen resistance in oestrogen receptor-positive breast cancer using the novel thiosemicarbazone anti-cancer agent, DpC. Br J Pharmacol 177:2365–2380. https://doi.org/10.1111/bph.14985
Mihailidou C, Papakotoulas P, Papavassiliou AG, Karamouzis MV (2018) Superior efficacy of the antifungal agent ciclopirox olamine over gemcitabine in pancreatic cancer models. Oncotarget 9(12):10360-10374. https://doi.org/10.18632/oncotarget.23164
Mody K, Mansfield AS, Vemireddy L et al (2019) A phase I study of the safety and tolerability of VLX600, an Iron Chelator, in patients with refractory advanced solid tumors. Invest New Drugs 37:684–692. https://doi.org/10.1007/s10637-018-0703-9
Oades RD (2010) The role of serotonin in attention-deficit hyperactivity disorder (ADHD). In: Handbook of Behavioral Neuroscience. Elsevier, pp 565–584
Poljak-Blazi M, Jaganjac M, Sabol I et al (2011) Effect of ferric ions on reactive oxygen species formation, cervical cancer cell lines growth and E6/E7 oncogene expression. Toxicol Vitr 25:160–166. https://doi.org/10.1016/j.tiv.2010.10.013
Qi S-S, Sun J-H, Yu H-H, Yu S-Q (2017) Co-delivery nanoparticles of anti-cancer drugs for improving chemotherapy efficacy. Drug Deliv 24:1909–1926. https://doi.org/10.1080/10717544.2017.1410256
Richardson DR, Kalinowski DS, Lau S et al (2009) Cancer cell iron metabolism and the development of potent iron chelators as anti-tumour agents. Biochim Biophys Acta - Gen Subj 1790:702–717
Saeki I, Yamamoto N, Yamasaki T et al (2016) Effects of an oral iron chelator, deferasirox, on advanced hepatocellular carcinoma. World J Gastroenterol 22:8967–8977. https://doi.org/10.3748/wjg.v22.i40.8967
Sahasrabudhe SR, Lai S, Pierce M et al (2008) Selective in vitro and in vivo anti-tumor activity of PRLX 93936 in biological models of melanoma and ovarian cancer. J Clin Oncol 26:14586–14586. https://doi.org/10.1200/jco.2008.26.15_suppl.14586
Scheers NM, Pereira DIA, Faria N, Powell JJ (2018) Ferric citrate and ferric EDTA but not ferrous sulfate drive amphiregulin-mediated activation of the MAP kinase ERK in gut epithelial cancer cells. Oncotarget 9:17066–17077. https://doi.org/10.18632/oncotarget.24899
Schulman HM, Hermes-Lima M, Wang E-M, Ponka P (1995) In vitro antioxidant properties of the iron chelator pyridoxal isonicotinoyl hydrazone and some of its analogs. Redox Rep 1:373–378. https://doi.org/10.1080/13510002.1995.11747014
Schulz TJ, Thierbach R, Voigt A et al (2006) Induction of oxidative metabolism by mitochondrial frataxin inhibits cancer growth: Otto Warburg revisited. J Biol Chem 281:977–981. https://doi.org/10.1074/jbc.M511064200
Seril DN, Liao J, Ho KLK et al (2002) Dietary iron supplementation enhances DSS-induced colitis and associated colorectal carcinoma development in mice. Dig Dis Sci 47:1266–1278. https://doi.org/10.1023/A:1015362228659
Seril DN, Liao J, Yang CS, Yang GY (2005) Systemic iron supplementation replenishes iron stores without enhancing colon carcinogenesis in murine models of ulcerative colitis: comparison with iron-enriched diet. Dig Dis Sci 50:696–707. https://doi.org/10.1007/s10620-005-2560-6
Silva AMN, Kong X, Hider RC (2009) Determination of the pKa value of the hydroxyl group in the α-hydroxycarboxylates citrate, malate and lactate by 13C NMR: Implications for metal coordination in biological systems. Biometals 22:771–778. https://doi.org/10.1007/s10534-009-9224-5
Spadaro M, Curcio C, Varadhachary A et al (2007) Requirement for IFN-γ, CD8+ T lymphocytes, and NKT cells in talactoferrin-induced inhibition of neu+ tumors. Cancer Res 67:6425–6432. https://doi.org/10.1158/0008-5472.CAN-06-4080
Stefánsson A (2007) Iron(III) hydrolysis and solubility at 25°C. Environ Sci Technol 41:6117–6123. https://doi.org/10.1021/es070174h
Su Y, Zhao B, Zhou L et al (2020) Ferroptosis, a novel pharmacological mechanism of anti-cancer drugs. Cancer Lett 483:127–136. https://doi.org/10.1016/j.canlet.2020.02.015
Tang D, Kroemer G (2020) Ferroptosis. Curr Biol 30:R1292–R1297. https://doi.org/10.1016/j.cub.2020.09.068
Torti SV, Torti FM (2013) Iron and cancer: More ore to be mined. Nat Rev Cancer 13:342–355
Torti SV, Torti FM (2020) Iron and cancer: 2020 Vision. Cancer Res 80:5435–5448. https://doi.org/10.1158/0008-5472.CAN-20-2017
Torti SV, Manz DH, Paul BT et al (2018) Iron and Cancer. Annu Rev Nutr 38:97–125. https://doi.org/10.1146/annurev-nutr-082117-051732
Toyokuni S (1996) Iron-induced carcinogenesis: The role of redox regulation. Free Radic Biol Med 20:553–566
Toyokuni S, Sagripanti JL (1993) Induction of oxidative single- and double-strand breaks in DNA by ferric citrate. Free Radic Biol Med 15:117–123. https://doi.org/10.1016/0891-5849(93)90050-5
Trujillo-alonso V, Pratt EC, Zong H, Lara-martinez A (2019) FDA-approved ferumoxytol displays anti-leukaemia efficacy against cells with low ferroportin levels. Nat Nanotechnol 14:616–622. https://doi.org/10.1038/s41565-019-0406-1.FDA-approved
Wang F, Elliott RL, Head JF (1999) Inhibitory effect of deferoxamine mesylate and low iron diet on the 13762NF rat mammary adenocarcinoma. Anticancer Res 19:445–450
Wang G, Zhao J, Zhang M et al (2019) Ferumoxytol and Cpg oligodeoxynucleotide 2395 synergistically enhance antitumor activity of macrophages against NSCLC with EGFRl858r/t790m mutation. Int J Nanomedicine 14:4503–4515. https://doi.org/10.2147/IJN.S193583
Weir SJ, Wood R, Schorno K et al (2019) Preclinical pharmacokinetics of fosciclopirox, a novel treatment of urothelial cancers, in rats and dogs. J Pharmacol Exp Ther 370:148–159. https://doi.org/10.1124/jpet.119.257972
Xie Y, Hou W, Song X et al (2016) Ferroptosis: process and function. Cell Death Differ 23:369–379
Zhang R, Wang F, Wang T (2020) The role of erastin in ferroptosis and its prospects in cancer therapy. Onco Targets Ther 13:5429–5441
Funding
Statutory Funds of the Department of Cellular and Molecular Biology, Faculty of Pharmacy, Wrocław Medical University No.:SUB.D260.21.095.
Author information
Authors and Affiliations
Contributions
Conceptualization: WS; Writing the original manuscript: WS, MG, KK, KR, AK, KW, JP, KC, ADJ; Supervision and founds acquisition: JS, JK.
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Szlasa, W., Gachowska, M., Kiszka, K. et al. Iron chelates in the anticancer therapy. Chem. Pap. 76, 1285–1294 (2022). https://doi.org/10.1007/s11696-021-02001-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11696-021-02001-2