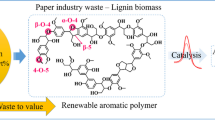
Abstract
Lignin is a potentially high natural source of biological aromatic substances. However, decomposition of the polymer has proven to be quite challenging, as the complex bonds are fairly difficult to break down chemically. This article is intended to provide an overview of various recent methods for the catalytic chemical depolymerization of the biopolymer lignin into chemical products. For this purpose, nickel-, zeolite- and palladium-supported catalysts were examined in detail. In order to achieve this, various experiments of the last years were collected, and the efficiency of the individual catalysts was examined. This included evaluating the reaction conditions under which the catalysts work most efficiently. The influence of co-catalysts and Lewis acidity was also investigated. The results show that it is possible to control the obtained product selectivity very well by the choice of the respective catalysts combined with the proper reaction conditions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Lignin is a natural high source of aromatic chemicals. It can be found together with lignocellulose and cellulose in wooden plants and is the most common biopolymer in nature. Lignin is a heterogeneous alkyl-aromatic polymer that can comprise up to 30–40% of the plant cell wall by mass, depending on the plant type (Wang et al. 2013). Its primary functions in nature are for structure, water transport, and defense against pathogens. Lignin is found in trees and other lignocellulosic plant-based materials. Currently, lignin is mainly a by-product of the paper industry. However, the use of lignin in renewable energy and for the production of aromatic chemicals is very promising (Kleinert und Barth 2008; Ouyang et al. 2020; Pandey und Kim 2011). As the demands for renewable materials have significantly increased in the last decade, exploitation of biomass is gaining importance (Calvo-Flores und Dobado 2010; Guigo et al. 2010). Lignin is typically used for gaining energy in combustion process (Windeisen und Wegener 2008; Kleinert und Barth 2008; Font et al. 2003). Merely, a small percentage is being used for regenerative purposes. Due to the complex structure of lignin (Scheme 1), it is extremely difficult and challengeable to break (Wang et al. 2013). Various approaches for the degradation of lignin have been investigated in the past decades from pyrolytic (Yang et al. 2007; Kawamoto 2017; Saiz-Jimenez und Leeuw 1986; Hosoya et al. 2007) up to biological methods with focuses on wood feeding insects (Geib et al. 2008) and fungi (Goodell 2003).
The catalytic chemical depolymerization of lignin is another promising method to generate small organic compounds, which can be used as basic chemicals in a wide range of industrial applications. Catalysts allow chemical conversions toward products with high stereo- and regioselectivity, as well as improved yields and positive effects on reaction conditions. In case of the depolymerization of lignin, where bonds are rather broken than formed, catalysts can have positive influence on product diversity and selectivity. Therefore, in this article, we would like to review the recent approaches of the past decade in this field of research with a focus on metal and zeolite catalysts including various cocatalytical combinations.
Nickel catalyst
Metal catalyst: general heterogeneous catalytic reaction using Ni/C catalyst
A highly promising approach to achieve the depolymerization of lignin is through the use of heterogeneous metal catalysts. These are capable of degrading the C–O and C–C bonds of lignin (Luo et al. 2016). Consequently, a high lignin conversion is obtained. Furthermore, it has been shown that these reactions progress under moderate conditions (Luo et al. 2016). Studies and research provide that particularly nickel-based catalysts are highly effective as well as selective in the depolymerization of lignin into phenolic monomers (Song et al. 2013). Highly effective outcomes were obtained with supported nickel (Luo et al. 2016). In order to investigate the efficiency of nickel supported on activated carbon catalysts (Ni/C), several experiments were assembled and evaluated. These are briefly summarized in the following.
For the purpose of investigating the influence of Ni/C catalyst of the degradation of lignin, various experiments at different reaction conditions were conducted using Miscanthus, also called silvergrass (plants from the grass family, mainly cultivated as ornamental plants) as biomass (Luo et al. 2016). Table 1 illustrates the influence of different reaction conditions on a catalytic lignin depolymerization using different amounts of Ni/C catalysts. For this purpose, 1 g of milled Miscanthus was added to 45 mL of methanol (MeOH). The mixtures were then heated for 12 h at 35 bar H2 at 225 °C (Luo et al. 2016). Under these reaction conditions, high yields were obtained particularly for the phenolic products 1–4. This is due to the Miscanthus, which is a grass species, so its lignocellulose contains mainly ferulate linkages (Luo et al. 2016). Therefore, the products 3 and 4 are characteristic products indicating these linkages. For this reason, higher yields are achieved by using Miscanthus than when conventional wood species are used (Luo et al. 2016). During the depolymerization of lignin in Miscanthus, a conversion yield to phenolic products of 68% can be observed. In the depolymerization of lignin obtained from wood species, only a conversion of 50% was achieved. This is due to the fact that the products 3 and 4 account for about 25% of lignin (Luo et al. 2016) (Scheme 2).
In the experiments, the effect of direct contact between the catalyst and the biomass on the reaction was investigated. Therefore, in the experiments 1–3, the Ni/C catalyst was separated from the biomass using a microporous cage (325 mesh, Luo et al. 2016). The experiments 4–6 were carried out under the same conditions as experiments 1–3. However, no microporous cage was used. The results show that quite similar results were obtained with both variants. This suggests that the lignin in the MeOH solution was depolymerized into oligomeric units and the soluble oligomeric fragments were then further converted to monomeric products by the Ni/C catalyst (Luo et al. 2016). In general, the variation of the catalyst had only little effect on the achieved overall yields or on the distribution of the phenolic products apart from entry 6 (5 wt% Ni/C) which showed significant amounts of compound 5 with significant decreased amounts of compounds 1 and 2 and slightly decreased amounts of compounds 3 and 4.
Other research also indicates that there is a dependence of the used biomass and the catalyst loading in terms of product scope and the monomer yields (Klein et al. 2015). This was demonstrated by a series of experiments in which three different feedstocks Birch, Poplar and Eucalyptus were added to 5 and 10 wt % Ni/C catalysts in MeOH (Klein et al. 2015). The results are summarized in Table 2 (Scheme 3).
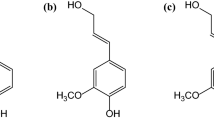
1. Dihydroeugenol.
2. Propylsyringol.
5. Isoeugenol.
For this purpose, 4.0 g of ground of the feedstock (birch, poplar and eucalyptus) was put in an ethanol(EtOH)–benzene mixture (1:2) for 12 h in a Soxhlet extractor (Song et al. 2013). Subsequently, 2.0 g of the treated crushed birch wood was placed together with 0.10 g of the catalyst in 40 mL of MeOH in a 75-mL autoclave reactor, which was then purged with Ar 4–6 times (Song et al. 2013). The reaction was then carried out at 200 °C for 6 h at a stirring speed of 500 rpm (Klein et al. 2015). For every substrate, experiments with 5 wt% Ni/C and with 10 wt% Ni/C were carried out. The results of the first three experiments show that birch wood seems to be more suitable for the depolymerization of lignin than the other used feedstocks, as it exhibits the highest lignin yield of 20%. This could possibly be attributed to the fact that birch wood contains a lower amount of xylan and that birch wood displays a higher uniformity of lignin referred to the various possible bonds in lignin, allowing a more selective catalytic depolymerization (Klein et al. 2015). The primary products of both birch and eucalyptus were compounds 5 and 6, with 8% and 12% for birch and 6% and 8% for eucalyptus, respectively. Experiments 4 to 6, using 10 wt% NiC catalyst loading, show a significant difference in the yields and in the products obtained. The product yields increased significantly for all three substrates. In particular, for the poplar and eucalyptus substrates, the yields progressed from 6 and 16% to 26% and 28%, respectively. The data suggest that in the presence of 10 wt % Ni/C, hydrogenation of the obtained products 5 and 6 result in the formation of the products 2 and 7 (Klein et al. 2015). Furthermore, a considerable increase in pressure was observed in experiments 4 to 6. This may be caused by the higher formation of H2 by the use of MeOH in the presence of 10 wt% Ni/C (Klein et al. 2015).
1. Dihydroeugenol.
2. Propylsyringol.
8. Alkene-substituted dihydroeugenol and propylsyringol.
9. Dimers.
Table 3 summarizes experiments conducted to examine the activity of Ni/C catalysts in the depolymerization of lignin using different kinds of solvents. For this purpose, 2.0 g of the pretreated birch sawdust and 0.10 g of the Ni/C catalyst were mixed with 40 mL of the used solvent at 200 °C and a stirring speed of 500 rpm for 6 h in an autoclave reactor. The reactor was then cooled to room temperature (Song et al. 2013). The first entry of the conducted experiments shows that without the use of the catalyst lignin cannot be depolymerized to phenolic products, as the various linkages cannot be cleaved. By using MeOH as a solvent a lignin conversion of 54% was obtained (Song et al. 2013). Furthermore, a total selectivity of products 8 and 9 of 89% can be achieved. Next, EtOH and ethylene glycol (EG) were used as solvents. The experimental data of entries 3 and 4 show extremely similar results both in lignin conservation, which is about 50%, and in the selectivity of the products. In the fifth entry, isopropanol (iPrOH) was used, and the experimental results show that it is less efficient than MeOH. It has a very low lignin dissolution capacity, resulting in a lignin yield of merely 27% (Song et al. 2013). An equally inefficient lignin yield of 16% was achieved when using glycerol aqueous solution (25 vol%). This is due to the high viscosity (Song et al. 2013). 1,4-dioxane has the property of dissolving lignin. 1,4-dioxane differs fundamentally from alcohols. For example, it lacks hydrogenation capabilities. This was evident in the fact that propenyl-substituted phenols were formed (Song et al. 2013). In entries 8 and 9, mixtures of MeOH and water were used as solvents. Both experiments demonstrate that the use of pure MeOH provides much better results. With both mixtures, only low lignin yields of 9% and 22% were obtained. The product selectivity was also affected and much lower. The experiments show that a higher MeOH content in the solutions results in a higher lignin yield (Song et al. 2013). In the tenth entry, cyclohexane was used, in which no lignin yield was obtained. This is due to the fact that possibly no hydrogen can be formed under the reaction conditions and that lignin has a very poor dissolubility (Song et al. 2013). It was observed that especially alcoholic solvents performed well. This can be attributed to the fact that the polar groups contained in the lignin interact with the alcohol molecules of the solvent, which promotes the depolymerization of lignin (Shu et al. 2015). Alcohols such as MeOH, EtOH, propanol and butanol are particularly suitable for this purpose because they exhibit a strong Lewis base (Shu et al. 2015). In addition, products such as monomers can dissolve excellently in alcohols. This also prevents repolymerization. The following equations were used to determine the yields (Song et al. 2013).
It can be concluded that several different factors influence the efficiency of a heterogeneous catalytic reaction using nickel supported on activated carbon catalysts. The usage of a microporous cage, in order to separate the catalyst and the biomass, showed only very little effects, whereas the use of the substrate is very important. Birch wood was shown to be more efficient in the depolymerization of lignin than the usage other feedstocks like eucalyptus. In addition, the choice of solvent is very crucial. A number of experiments have shown that MeOH is particularly suitable for this purpose (Table 4).
Heterogeneous catalyst RANEY Nickel
The use of RANEY nickel as a catalyst has been shown to support the production of phenols (Forchheim et al. 2012). Besides achieving a high phenol yield, another benefit of using Raney nickel is that they are very low cost (Zhao et al. 2010). Raney nickel is capable of serving as a catalyst in the hydrogenolysis of ether bonds (Wang und Rinaldi 2012). In order to demonstrate the influence of the heterogeneous catalyst RANEY nickel on the degradation of lignin various experiments were conducted and are presented in the following. For this purpose, experiments investigating the process of hydrogenolysis were performed. Thereby, the effect of the used solvent and the resulting products were analyzed. Furthermore, the obtained product selectivity was observed by executing hydrothermal lignin degradation experiments.
Hydrogenolysis of diphenyl ether with Raney Ni conducted in several solvents
10. Cyclohexyl phenyl ether.
11. Dicyclohexyl ether.
12. Phenol.
13. Benzene.
14. Cyclohexanol.
15. Cyclohexane.
For the hydrogenolysis of diphenyl ether, 100 mg of RANEY nickel, 2.9 mmol of diphenyl ether and 15 mL of the used solvent were added in a 30-mL batch reactor, which was then purged with hydrogen (Wang und Rinaldi 2012). Afterward, the reactor was heated from an initial pressure of 50 bar H2 at 25 °C to 90 °C for 10 min. The reaction took 2.5 h and was conducted at a stirring rate of 300 rpm. Afterward, the obtained products were analyzed by using GC-FID and GC–MS (Wang und Rinaldi 2012).
The experiment has shown that the reaction is significantly influenced by the used solvent. Using alcohols as solvents, iPrOH in particular was found to be effective, with a conversion of 72.7%, followed by 2-butanol, which yielded a conversion of 55.1%. Worse results were achieved by using EtOH, 1-butanol and tert-butanol. The worst outcome was obtained using MeOH, with a conversion of only 12.4% (Wang und Rinaldi 2012). Moderate results were obtained using 2-Me-THF with a conversion of 61.1% and ethyl acetate with a conversion of 47.0%. In contrast, the use of tetrahydrofuran and 1,4-dioxane achieved poor results with conversions of 29.6% and 17.8%, respectively (Wang und Rinaldi 2012). By using alkanes as solvents, excellent results were achieved. This was evident from the fact that when n-heptane and decalin were used, almost complete conversions of 99.0% and 99.6%, respectively, were achieved. By using methylcyclohexane (MCH), a conversion of 100% was realized. An equally great outcome was observed by using 1,1,1,3,3,3-Hexafluoro-2-propanol (Hex-F-2-PrOH), in which the substrate was likewise completely converted (Wang und Rinaldi 2012). The use of RANEY nickel is favorable as it allows the hydrogenolysis of ethers and the hydrogenation of arenes (Wang und Rinaldi 2012). The experiments on phenol/cyclohexanol and benzene/cyclohexane ratios indicate that the type of solvent can enhance selectivity (Wang und Rinaldi 2012). The highest aromatic yield was achieved by using MeOH and 1,4-dioxane. This was reflected in the high yields of phenol and benzene. The use of alkanes and hex-F-2-PrOH, on the other hand, provided extremely poor results with almost no resulting aromatic yields. The results suggest that the formation of aromatics occurs only by hydrogenolysis of the ether bond. RANEY nickel is suitable for catalyzing the hydrogenation of phenol to cyclohexanol, but for the conversion of phenols to alkanes, an additional acid catalyst is required (Wang und Rinaldi 2012) (Scheme 4).
Spruce lignin hydrogenolysis
The gas–liquid chromatography of spruce lignin hydrogenolysis products in which Raney nickel, ruthenium on charcoal and ruthenium on alumina was used as catalysts revealed the formation of nine compounds. Among them, four compounds were identified as aromatic derivatives and five as cyclohexyl derivatives (Pepper and Lee, 1970). A pure sample was taken from the gas chromatograph for each compound. They were then compared to an accurate sample for their relative retention time and their mass. Infrared (i.r.), and nuclear magnetic resonance (n.m.r.) spectra (J. M. Pepper and Y. W. Lee). The relative abundance of the lignin degradation products obtained was determined for all three catalysts used. For this purpose, dihydrosinapyl alcohol was used as an internal standard (J. M. Pepper and Y. W. Lee). The abundances were then reported in terms of the weight percentage of the total ether-soluble fraction (J. M. Pepper and Y. W. Lee). Both a planimeter and the triangulation method were used for the determination. The average of the values obtained was then used (J. M. Pepper and Y. W. Lee). In the following experiment, lignin degradation was achieved by a hydrogenolysis process. For this purpose, 10 g of extracted spruce wood flour was heated in dioxine water (1:1 v/v, 150 mL) at a temperature of 195 °C and at an starting hydrogen pressure of 34.5 bar for 5 h (J. M. Pepper and Y. W. Lee). This process was repeated using the individual catalysts. In the first experiment, 10 g of Raney nickel was used (J. M. Pepper and Y. W. Lee). An F&M chromatograph of the model 5750 was used for the study. The dimensions of the column were 1/8 inch, 6 feet and it was filled with 10% SE-30 Chromosorb W. In addition, the helium flow rate was 40 mL/min (J. M. Pepper and Y. W. Lee). The temperature was heated from 100 to 250 °C at a rate of 4°/min. The results of the study are provided in Table 5. They show that the use of the catalysts leads to an increased lignin degradation. In addition, a ring reduction to the corresponding cyclohexyl derivatives could be observed (J. M. Pepper and Y. W. Lee). The main components obtained were those containing a 1-substituted-3-propanol structure (J. M. Pepper and Y. W. Lee). Compared to the ruthenium catalysts supported on char coal or alumina, the use of Raney nickel as a catalyst achieved the highest lignin yield of 16.5% (J. M. Pepper and Y. W. Lee). The use of Raney nickel particularly favored the recovery of the 3-(4-hydroxycyclohexyl)-1-propanol component. (J. M. Pepper and Y. W. Lee) (Scheme 5).
Hydrothermal lignin degradation experiments
12. Phenol.
18. 4-methylguaiacol.
25. 4-Methyl-catechol.
26. Methane.
27. C2–C4
28.Carbon dioxide.
For the hydrolysis of lignin, 3 mL of water, 0.4 g of lignin (spruce) and 0.1 g of RANEY nickel were added to a 5-mL microbatch reactor and heated at varying temperatures and time periods for different experiments (Forchheim et al. 2012). The used spruce wood was prior subjected to the simultaneous saccharification and fermentation process. The gaseous and organic phases were analyzed using gas chromatography (Forchheim et al. 2012). The experiment demonstrated that RANEY nickel as a catalyst affects the selectivity of phenol (12). This effect can be seen in particular in the experiments carried out at a temperature of 633 K (entries 5–7) (Forchheim et al. 2012). Here, a relatively high phenol (12) yield was obtained, whereas the yield of guaiacol (23) and catechol (24) remained rather low. The formation of phenol (12) as well as p-cresol (22) could be strongly supported by increasing the temperature as well as the reaction time. However, these changes led to a decrease in the formation of the products guaiacol and catechol (Forchheim et al. 2012). In addition, the impact of the reaction conditions on the yield of the solid residue could be evaluated. In general, increasing the temperature and reaction time resulted in a decrease in yield. In the experiments that lasted 1200 min (entries 4 and 10), the yield equaled 0% (Forchheim et al. 2012). The study has shown that Raney nickel promotes gasification under certain reaction conditions (Forchheim et al. 2012). In particular, the experiments with an increased temperature showed a high gas formation. Carbon dioxide was formed most frequently, followed by the formation of methane. In addition, a small amount of hydrocarbon gases was produced. It could be observed that the formation of all mentioned gases was favored by an increase in the reaction temperature and time (Forchheim et al. 2012). Furthermore, it was observed that by the presence of RANEY nickel catechol was converted to phenol (12). Cyclohexanol (14), on the other hand, was not formed. This could possibly be due to the fact that the temperature is unsuitable for this purpose and that an insufficient amount of hydrogen is present (Forchheim et al. 2012). The formation of phenol was the result of liquefaction. The experiment has shown that RANEY nickel is not able to induce the formation of monoaromatic compounds. However, the use of RANEY nickel as a catalyst leads to the hydrogenation of intermediate products of lignin degradation such as guaiacol and catechol from which phenol can be obtained (Forchheim et al. 2012). Since high temperatures and long reaction times increase the formation of phenols, it is expected that a high phenol yield requires a considerable amount of time. Since the increase in temperature and time also results in gasification, it is likely that the phenol yield will eventually decrease over extended reaction times (Forchheim et al. 2012) (Tables 6 and 7).
By increasing the temperature of the reaction, the obtained product selectivity can be diversified. Furthermore using RANEY nickel, the gasification of guaiacol and catechol is favored by a higher reaction temperature, which results in the generation of aromatic products. However, very high temperatures promote the gasification of guaiacol. The formation of gas results in a lower aromatic yield. The formation of solid residue is suppressed near critical water, as RANEY nickel suppresses the formation of intermediates that can lead to polymerize (Forchheim et al. 2012). It can be concluded that solvents are able to direct the product selectivity. Therefore, it must be considered wisely which solvent is best suited for conversion of lignin by hydrogenolysis of C–O ether bonds (Wang und Rinaldi 2012).
Nickel Zeolite
In all three experiments, a 300-mL batch reactor was used. 0.5 g of Ni catalyst and 1 g of organosolv lignin and 100 ml (77 g) of hexadecane solvent were added to the reactor. 1 ml of n-heptane was used as an internal standard. The reactor was then purged 3 times with hydrogen. Afterward, it was heated at a pressure of 20 bar H2 to 523 K or 593 K within 15 min (Kasakov et al. 2015). The used stirring rate was 700 rpm. Finally, the reaction was terminated by cooling to room temperature. Different acidic zeolites have been used such as HZSM-5, which has a monoclinic framework (Vankoningsveld et al. 1990) and HBEA. To prepare the catalysts for the reaction, 2 g of zeolite and 210 mL of a 0.14 M Ni(NO3)2 solution were used. Also, 10.2 g of urea was dissolved in 40 mL of the Ni(NO3)2 solution. The urea nickel solution was added to the nickel zeolite solution at a temperature of 343 K. Subsequently, the temperature was heated to 363 K at and then after 3 h cooled to the ambient temperature. The solution was added to the nickel zeolite solution. The suspension was additionally vacuum-filtered. The resulting solid was rinsed 3 times with ultrapure water and incubated overnight at 383 K. The same procedure was performed to prepare the silica catalyst; however, the suspension was cooled after 3.5 h. The resulting gas was analyzed using Agilent Technologies 7890B GC and thermal conductivity and flame ionization detectors (TCD and FID). The collected liquid phase was also analyzed using an Agilent Technologies 7890B GC. The yield was determined using the following equation:
Using Ni/SiO2 as a catalyst led to a gasification of 4.1% of the lignin. On the contrary, 23% liquid phase products were obtained. Among these, 20% consisted of monocyclic alcohols and 80% of monocyclic alkanes. Only the Ni/SiO2 catalyst was able to recover monocyclic alcohols. For Ni/HZSM-5, a gas yield of 5.5% and a liquid products yield of 28% were generated. A total of roughly 90% monocyclic alkanes were recovered. Compared with the other catalysts which were used, the highest quantity of monocyclic alkanes was produced. The remaining 10% of the liquid products consisted of bicyclic alkanes. The reaction catalyzed with Ni/HBEA showed the highest yields of both gas phase products with 6.8% and liquid phase products with 35%. The reaction catalyzed with Ni/HBEA showed the highest yields of both gas phase products with 6.8% and liquid phase products with 35%. It was possible to obtain 83% of monocyclic alkanes and 17% of bicyclic alkanes. Therewith, the largest amount of bicyclic alkanes could be recovered.
The experiments demonstrated that zeolite-based nickel catalysts are suitable for lignin degradation and that it is possible to recover a large amount of monocyclic alkanes. Based on the results obtained, it is assumed that the HZSM-5 prevents the alkylation process of aromatic monomers due to its medium-sized pores. This can be explained by the fact that alcohols or olefins are formed during hydrogenation. This in turn leads to a low yield of bicyclic alkanes when HZSM-5 is used.
Furthermore, it was examined to what degree the temperature during catalysis impacts on the yield and the selectivity. For this purpose, the catalytic reaction with Ni/HBEA catalyst was carried out at different temperatures with the same reaction conditions as above described. The results are recorded in Table 8. In general, it was observed that with higher temperature, both the hydrocarbon yield and the product selectivity increased. Simultaneously, the amount of unconverted lignin and solid residue decreased. This outcome can be explained by the fact that at higher temperatures, the hydrogenation of phenols is accelerated. Therefore, a larger quantity of lignin is converted.
Zeolite Catalyst
In a series of experiments involving the depolymerization of kraft lignin, various catalysts were tested regarding their efficiency. The two low-cost catalysts Ni10%/zeolite and FHUDS-2 (a W-Mo-Ni industrial catalyst) were tested for their efficiency, and their performance was compared with that of a high-cost Ru catalyst (Table 9).
For this purpose, 5 g of the kraft lignin, 0.5 g of the nickel-based catalyst, 3 mL of formic acid and 30 mL of a 1:1 v/v water-EtOH solution were added to a reactor (Huang et al. 2017a). Subsequently, the reactor was purged 3 times with nitrogen. Afterward, the reactor was heated at a rate of 10 °C per minute to temperatures between 200 and 300 °C at a pressure of 2 MPa N2. The reactor was then purged 3 times with nitrogen. The stirring speed during this process was 290 rpm. After 1 to 3 h, the reaction was terminated by cooling the reactor in an ice bath. The resulting gases were analyzed by micro-GC. The solid residue was obtained from the liquid phase by filtration and vacuum drying overnight. The solid residue was later incubated in an oven at 105 °C for 24 h. The same procedure was performed using the Ru catalyst. However, instead of 0.5 g, only 0.25 g of catalyst was used. Since noble metal catalysts tend to have a higher activity than metal catalysts such as nickel-based catalysts. In addition, the catalyst is considerably more expensive.
The catalysts were compared with regard to their yield in terms of lignin depolymerization and solid residue. Initially, no catalyst was used. A large part of the lignin was still depolymerized, even though no catalyst was present. It can be concluded that the solvents used, formic acid and the water-EtOH mixture, can cause effective depolymerization of lignin at temperatures of 200–300 °C. All catalysts had almost no effect on the depolymerization as well as on the solid residue yield. This was observed at all temperatures. However, the use of catalysts showed effects on the reduction of the molecular mass with regard to the depolymerization yield as well as an increase in the molecular mass regarding the solid residue yield. This could presumably be due to the fact that the acidity of zeolites triggers a condensation of lignin on the surface of the catalyst. This effect was particularly evident at lower temperatures. The Ni10%/zeolite cold catalyst achieved the best results. For example, at 200 °C, the molecular mass was reduced to 3150 g/mol from 10 000 g/mol. The FHUDS-2 catalyst, on the other hand, achieved only 6180 g/mol and the Ru5%/C 3640 g/mol. The increase in the temperature shows an increase in the solid residue yield in all experiments; therefore, it can be assumed that the depolymerization is thermodynamically better at higher temperatures (Huang et al. 2017a).
In addition, the gas yield at different temperatures was also investigated with regard to the catalysts. The results are summarized in Table 10. It can be observed that the use of the Ru catalyst causes an increase in the production of the gases H2 and CO2. The increase in temperature also increases this effect. The lowest yields of the gases H2 and CO2 were recorded with the Ni/zeolite catalyst. It is noteworthy that almost no CO2 was produced, regardless of the temperature. This could be attributed to the fact that nickel and zeolite cause extremely strong adsorption to hydrogen and carbon dioxide.
Catalytic fast pyrolysis with ZSM-5 zeolites yields
When using zeolite-based catalysts, the SiO2/Al2O3 ratio of the respective catalyst plays a major role in terms of catalytic efficiency. Among other things, the ratio indicates the acid content of the catalyst. The following study dealt with this matter. For this purpose, the same experiment was carried out using differently desilicated catalysts. The properties of the catalysts are listed in Table 11. Lignin, which was obtained from birch wood powder, was used. In each case, 1 g of the ZSM5 zeolite catalyst (zeolite catalyst with an orthorhombic framework) was heated with 10 mL of NaOH solution at a temperature of 70 °C for a period of 2 h (Li et al. 2014). It should be noted that concentrations of 0.1–0.5 mol were used in the NaOH solution to ensure a different degree of desilication of the zeolite. The catalysts were then filtered and washed three times with deionized water. They were afterward dried overnight at a temperature of 110 °C. The catalysts were then treated with 1 M deionized water. This was followed by treatment with 1 M NH4NO3 solution at 80 °C, which replaced the zeolite ions. This was subsequently followed by drying again at 110 °C overnight. The catalysts then had to be calcined at 550 °C for 10 h. This resulted in an exchange of Na+ for H+ (Li et al. 2014). For the upcoming catalytic fast pyrolysis, the zeolite catalysts had to be calcined at 550 °C for 5 h in a muffle furnace. After that the zeolite catalysts could be mixed with the biomass in a ratio of 10:1, so that the pyrolysis could be carried out in a semi-batch reactor. 4 mg each of the catalyst biomass mixture was used for this purpose. During the fast pyrolysis, the temperature was heated to 650 °C. Helium was also used as the gas. After the pyrolysis was completed, the resulting products were analyzed using a gas chromatograph (Li et al. 2014). The selectivity of the desanded aromates was given as moles of carbon in the product in relation to the total moles of aromatic carbon. The following equation was used for the calculation:
The results of the pyrolysis are shown in Table 12. In addition, Table 11 shows that the alkaline treatment caused structural changes in the catalysts. Thus, it can be observed that the SiO2/Al2O3 ratio of the zeolites decreased with increasing concentration of the NaOH solution used for the alkali treatment. The Si molecules were thus removed from the zeolite. The original zeolite catalyst has a comparatively high Brønsted acidity and much fewer Lewis acid sites (Li et al. 2014). Exposure to NaOH significantly reduced the amount of Brønsted acid sites on the zeolites. In addition, it is assumed that mesopores were also formed which additionally enhanced the catalytic activity, since the diffusion path was improved. The results show that a higher yield of phenols and aromatics was achieved by desilication. In addition, lower coke yields were achieved. This indicates that the desilicated zeolites exhibit a higher catalytic activity in the conversion of oxygenates to aromatics. This improvement can be explained, on the one hand, by the fact that the density of the Brønsted acid sites in the zeolites decreases as a result of the desilication, whereas the accessibility of the acid sites increases (Li et al. 2014). Excessive desilication, such as was the case when the zeolite was treated with 0.5 M NaOH solution, led to an increase in mesoporosity and a decrease in the microporosity of the zeolite, which worsens the diffusion path. As a result, the conversion of biomass-derived oxygenates to aromatics is poorer (Li et al. 2014).
Effect of desilication on ZSM-5 zeolite and in catalytic fast pyrolysis
13. Benzene.
29. Toluene.
30. Ethylbenzene.
31. p,m-Xylene.
32. o-Xylene.
33. 1,2,3-Trimethylbenzene.
34. Indene.
35. Naphthalene.
36. 2-Methylnaphthalene.
37. 1-Methylnaphthalene.
38. 1,7-Dimethylnaphthalene.
39. 2,6-Dimethylnaphthalene.
40. 1,8-Dimethylnaphthalene.
41. Anthracene.
In the subsequent experiment, pyrolysis was used to induce depolymerization. Since this involves breaking down the source materials lignin and the crude biomass into larger fractions which are often too large to fit into the existing pores of the zeolite catalyst, which is why the aim of the experiment is to investigate whether the catalytic activity can be improved by creating additional mesopores in the zeolite catalyst (Hoff et al. 2017). In order to achieve this, two pyrolysis experiments were carried out, one using a ZSM-5 catalyst and the other using a ZSM-5 catalyst previously treated with a 0.2 M NaOH solution. The NaOH treatment caused the formation of mesopores on the catalyst surface (Hoff et al. 2017). To accomplish this, a micro-pyrolyzer equipped with an auto-shot sampler was used. The biomass and the zeolite catalyst were first mixed in a ratio of 1:20 (Hoff et al. 2017). Then, 5 mg of each mixture was added to the pyrolysis furnace. The furnace was preheated to 550 °C beforehand. Subsequently, the vapor generated during pyrolysis was sucked into the gas chromatograph by using helium, and the gases were subsequently separated. The pyrolysis gas was analyzed using a mass spectrometer detector (MSD) or a flame ionization detector (FID) (Hoff et al. 2017). The results of the experiment were defined as follows. Product distribution was reported as molar carbon yield, which was defined as the molar ratio of carbon in a certain product to carbon in the starting material. The selectivity for aromatics was specified as moles of carbon in a distinct aromatic hydrocarbon in relation to the overall moles of carbon present in the fluid products. Desilication of the zeolite catalyst was performed by mixing 2 g of the ZSM-5 catalyst with a SiO2/Al2O3 ratio of 23 with 50 mL of NaOH solution in a 125-mL Nalgene flask. The flask was heated in an oil bath at 65 °C for 75 min. Meanwhile, the solution was stirred at 500 rpm using a magnet. The reaction was then terminated by cooling the flask in an ice bath for 10 min. The mixture was then centrifuged for 30 min. The obtained solid was rinsed with deionized water so that the liquid phase reached a pH value of 9. The sample was then stored in 50 mL of 0.1 M HCl solution at 65 °C for 4 min (Hoff et al. 2017). The sample was subsequently rinsed once more with deionized water until the pH of the fluid equaled 5. Next, the sample was stored in 100 mL of 0.2 M NH4NO3 solution overnight. Finally, the samples were exposed to calcination at 550 °C for 10 h. The N2 adsorption–desorption isotherms based on which the pore size distribution, the total surface area and the micro- and mesopores were determined were measured by Micromeritics ASAP 2020 system at 77 K. The N2 adsorption–desorption isotherms based on which the pore size distribution, the total surface area and the micro- and mesopores were determined were measured by Micromeritics ASAP 2020 system at 77 K. To investigate the SiO2/Al2O3 ratio, energy dispersive X-ray spectroscopy (EDS) was performed with an AztecTM spectrometer system (Hoff et al. 2017). The results on the catalyst properties are given in Table 13. Characterization of Brønsted and Lewis acid sites was performed by using NH3 desorption (NH3-TPD) and diffuse reflectance infrared Fourier transform spectroscopy (DRIFTS) (Hoff et al. 2017). This was done for various temperatures. The results are given in Table 14. Experimental data of the pyrolysis test are given in Table 15. The results of the experiment showed that for lignin, the 0.2 M catalyst reduced the aromatics yield and that in the case of pyrolysis of red oak, the aromatics yield was increased.
The selectivity of the pyrolysis products can be controlled by the choice of a compatible catalyst. The ZSM-5 catalyst is excellently suited for this purpose, since it allows the highest selectivity for monocyclic platform aromatics to be achieved. However, despite this, a large portion of the carbon of the feedstock is converted to coke or coal. These products are undesirable by-products whose production should be reduced. The formation of coke is promoted on the one hand by condensation reactions in which polyaromatic hydrocarbons are formed which take place in the micropores (Hoff et al. 2017). On the other hand, this is favored by the formation of smaller oxygen compounds which are polymerized on the outer surface of the zeolite. Furthermore, it has been shown that during pyrolysis, up to 30% of the carbon is lost due to the above-mentioned processes (Hoff et al. 2017). The formation of coke can be reduced to a large extent by improving the diffusion as well as by more easily accessible bulk acid sites. This also leads to a better product selectivity. The performance of a catalyst is limited by insufficient diffusion pathways. When the pores are widened, the diffusion rate is immediately improved, increasing the catalytic activity for many reactions (Hoff et al. 2017). In addition, the formation of an outer film can restrict mass transfer by up to 60%. Therefore, desilication is a suitable method to promote intracrystalline diffusion and mass transfer, thus making the active sites more accessible. For ZSM-5 catalysts, the aluminum concentration has to be taken into account. Zeolite catalysts, which have a fairly high aluminum content, are quite resistant to the alkaline treatment (Hoff et al. 2017). This can cause structural changes in the ZSM-5 catalyst, which in turn can have a serious effect on the reaction and product selectivity. Among other things, the yield of aromatic products can be reduced by up to 50% (Hoff et al. 2017).
The experiment demonstrated that the zeolite catalyst was desilicated by NaOH, resulting in an increase in the mesoporosity. In the fast pyrolysis of cellulose, lignin and red oak it became evident that only in the pyrolysis of cellulose by the treated catalyst an improvement in the mesoporosity could be achieved. It was found that especially in the pyrolysis of lignin and red oak, a catalytic improvement was achieved by the desilicated catalyst. It is assumed that this is related to a simplified diffusion and a more efficient mass transport. The alkali treatment, besides creating mesopores, needs to facilitate Brønsted acid sites, so that the deoxygenation and conversion of bulky reactants to aromatic hydrocarbons is enhanced (Hoff et al. 2017).
Zeolite catalyst acidity and structure of ZSM-5
The following study investigates the influence of the starting material as well as the acidity of a zeolite catalyst in the depolymerization of lignin by pyrolysis, which was also carried out at three different temperatures. The pyrolysis was performed with raw lignin as well as with torrefied lignin. In all experiments, the catalyst/feed ratio amounted to 1 to 4 (Adhikari et al. 2014). The ZSM-5 catalysts were calcined beforehand in order to obtain different acid contents. The catalysts were then mixed together with the respective lignin types in a ratio of 1:4. The pyrolysis gas used was helium (Adhikari et al. 2014). The gas resulting from the pyrolysis was then transferred to a gas chromatograph. The liquid products were analyzed using an Agilent 7890 GC/5975 MS (Adhikari et al. 2014). The results of the experiment are shown in Table 16. The properties of the differently calcined catalysts are given in Table 17. Furthermore, the following equation was used to calculate the carbon yield:
Untreated raw lignin was firstly used as the starting material. It was found that a large amount of hydrocarbons could be recovered by using a catalyst in all experiments. It can be concluded that the zeolite catalyst enhances the direct deoxygenation of methoxyphenols to aromatic hydrocarbons (Adhikari et al. 2014). In addition, the use of the catalyst enhances the formation of olefins. It can therefore be assumed that the aliphatic bonds in the lignin are increasingly broken. This is positive since olefins are converted into aromatic hydrocarbons by the aromatization reaction (Adhikari et al. 2014). With regard to the acidity of the catalyst, it was found that the formation of aromatic hydrocarbons was also boosted when the acidity increased. Regarding the temperature, it was found that only at lower temperatures, such as 500 °C, the formation of guaiacols took place only using less acidic catalysts such as Z80 and Z280. From this, it can be concluded that ZSM-5 catalysts with higher acidity are more suitable for the cleavage of aromatic C–O bonds in lignin. The highest yield of phenolic products was obtained with the Z280 catalyst. This catalyst exhibits the lowest acidity. In general, it was observed that due to the temperature deviations of the pyrolysis, there were only minor differences in the yield of aromatic products (Adhikari et al. 2014).
Subsequently, the same experiment was performed again using torrefied lignin as the starting material. In this case, likewise as in the pyrolysis of the raw lignin, mainly aromatic hydrocarbons were obtained. It was observed that the higher acid content of the catalyst increased the yield of aromatics. The use of catalyst Z30, which has an extremely high acid content, also resulted in an increased phenol yield (Adhikari et al. 2014). Compared to crude lignin, hardly any guaiacols were formed during the pyrolysis of the torrefied lignin, even with the less acidic catalysts. It can therefore be assumed that the torrefaction process leads to structural differences that favor the deoxygenation efficiency of the zeolite catalyst. The experiment has demonstrated that the acidic zeolite catalysts (in particular the Brønsted acid sites) increase the cleavage of bonds such as methoxyl units found in guaiacols (Adhikari et al. 2014). This is mainly due to the fact that they release protons, which allows the formation of aromatic compounds and phenols. The highest yields of aromatics were obtained with the particularly acidic catalysts Z30 and Z50 at temperatures of 500 °C and 550 °C, respectively. For the less acidic zeolite catalysts, on the other hand, no great difference was obtained due to the temperature difference. It can be concluded from the experiment that both the choice of starting material and the acidity of the catalyst have an impact on the product selectivity. Increased acidity and the torrefracturing process resulted in better yields of aromatics and phenols (Adhikari et al. 2014) (Scheme 6).
H-ZSM-5 and H-beta comparison
42. Acetic Acid.
43. Furfural.
44. Furfural alcohol.
45. Hydroxyacetone.
48. p-xylene.
49. o-xylene.
The following study compared the two zeolite acidic catalysts H-Beta and H-ZSM-5 with respect to their product selectivity. The different properties of the two catalysts are listed in Table 18. Different lignin-containing materials were used for this purpose. In addition, the effect of the acid content of the catalyst was also tested. For this purpose, a Frontier Lab Double-Shot micro-pyrolyzer PY-2020iD equipped with the Frontier Lab Auto-Shot Sampler AS-1020E was used (Mihalcik et al. 2011). This is also connected to a gas chromatograph. The products generated by the pyrolysis were identified by a mass spectrometer. About 1 g of the respective biomass with 5 mg of the catalyst was added to the pyrolysis apparatus.
The results of the test in which the H-ZSM-5 catalyst was used are displayed in Table 19. It can be seen that the H-ZSM-5 catalyst performed best, as it significantly minimized the oxygenated compounds. This can be observed with all the feedstocks used. The product selectivity, however, was significantly influenced by the feedstock. In general, the catalyst H-ZSM-5 (23) exhibits a strong bond cleavage property, which is why only very small amounts of hydroxyacetone (45), acetic acid (42) and syringol (47) could be identified. In addition, the best yield was obtained with aromatic hydrocarbon. Substituted benzenes and naphthalenes were increasingly abundant. Looking at the Si/Al ratios, it was found that the H-ZSM (280) catalyst generated the least aromatics, while the H-ZSM-5 (23) catalyst generated the most aromatics. It can be concluded that the acidity of the catalyst has a strong effect on the formation of aromatics (Mihalcik et al. 2011).
The results of the experiment in which H-beta catalysts are given in Table 20. It was found that in terms of aromatics yield, the H-beta catalysts produced a higher conversion than the H-ZSM (50, 280) catalysts for all feedstocks used.
The test demonstrated that all catalysts used were able to reduce the oxygen within the pyrolytic vapors. The H-ZSM-5 (23) catalyst showed the best results. This catalyst also achieved the highest yield of aromatics. This shows that the ratio of Si and Al has a significant effect on the catalytic activity. Since this enhances the properties to the deoxygenate, more aromatics could be obtained (Mihalcik et al. 2011).
Cu–Mo doped zeolite ZSM-5 catalyzed conversion
In this study, the three zeolite catalysts Cu/Mo-ZSM-5, Cu-ZSM-5 and HZSM-5 were tested under different reaction conditions. This involved testing different water–MeOH ratios for the solvent. The properties of the individual catalysts are shown in Table 21. The results of the experiment are shown in Table 22. To accomplish this, 0.5 g each of lignin and 0.125 g of zeolite catalyst were heated with 60 ml of 1.7 mmol NaOH solvent in a 100-ml reactor at 220 °C for 7 h (Singh und Ekhe 2015). Prior to this, the reactor was purged with argon 3 to 5 times. After the reaction was terminated, the obtained products were washed with 20 mL of water. Subsequently, centrifugation was performed at 2500 rpm for 15 min. This allowed separation of the liquid and solid products. Ethyl acetate (EtOAc) was used to extract the reaction products present in the organic and aqueous phases (Singh und Ekhe 2015). The catalyst, the resulting char and fragments of lignin could not be dissolved in EtOAc (Singh und Ekhe 2015). The products dissolved in ethyl acetate were then analyzed qualitatively and quantitatively. The yields were calculated using the following equations:
The experiment illustrates that by using NaOH as a solvent, a high lignin conversion could be achieved. This was backed up by the fact that in entry 6, when no NaOH was present in the solvent, a comparatively lower lignin yield was achieved. Furthermore, when only water and MeOH were used as solvents, a lower lignin conversion was also obtained. The solubility of lignin was evidently improved by the addition of NaOH. However, lignin is insoluble in water and very poorly soluble in MeOH, making them unsuitable solvents (Singh und Ekhe 2015). Furthermore, exposure to NaOH caused less formation of char, which is an undesirable by-product. The ratio of water to MeOH of 30:30 mL-1 as well as of 45:15 mL-1 was most suitable for the depolymerization of monomers. With regard to the catalyst, the Cu/Mo-ZSM-5 catalyst performed particularly well, since both high lignin conversion rates and high monomer yields were achieved (Singh und Ekhe 2015). In all tests, it performed better in this respect than the Cu-ZSM-5 catalyst. In addition, the Cu/Mo-ZSM-5 catalyst was recycled and successfully used for three cycles without any significant difference in efficiency. In terms of both lignin conversion and monomer yield, the highest conversions were obtained with the HZSM-5. However, it was negative that no high product selectivity for a phenol was formed. Only higher amounts of emulsified phenols and hydro-deoxygenated products such as acyclic hydrocarbons and aromatics were obtained. The catalyst could be recovered and reused after the reaction (Singh und Ekhe 2015). However, the quality of the catalyst continued to decrease after each cycle was used. The catalyst could be used for a total of three cycles with loss of quality (Singh und Ekhe 2015).
Palladium catalyst
Tandem-catalyzed reductive depolymerization with metal triflate and Pd/C
In the subsequent experiment, the catalytic performance in the degradation of ligneous biomass by Pd/C catalysts in combination with various metal triflates was investigated. The results are shown in Table 23. Pd/C catalysts are mainly involved in the cleavage of α-O-4, 4-O-5 and β-β bonds of the aromatic lignin framework (Huang et al. 2017b). Metal triflates are responsible in the splitting of ester and ether linkages as well as the β-O-4 ether bonds (Huang et al. 2017b). Therefore, in the degradation of lignin, optimizing Pd/C catalysts using metal triflates is an excellent application, as they become active in the cleavage of different types of bonds. The combination enables the promotion of the reductive fractionation process. In order to perform such a procedure, 2000 mg of extracted birch wood sawdust (125–300 μm) and 200 mg of 5 wt% of the Pd/C catalyst as well as 0.0322 mmol of each of the cocatalysts were loaded into a reactor together with 40 ml of the MeOH solvent (Huang et al. 2017b). All these components were mixed at 30 bar hydrogen at a stirring speed of 500 rpm for one and two hours, respectively. In addition, a comparison was made of the influence of the replacement of nitrogen with hydrogen on the reaction. For this purpose, the experiment with the co-catalysator Yb(III) triflate was carried out under the same conditions with nitrogen and hydrogen. To analyze the obtained liquid products, a Shimadzu 2000 GC–MS system equipped with a RTX-1701 column, a flame ionization detector (FID) as well as mass spectrometer (MS) detector was applied (Huang et al. 2017b). To determine the lignin yield of the monomers, the following equation was used:
To quantify the sugars, the obtained hydrolysates were first diluted 5 times with a solution containing the concentration of the internal standard fucose. Subsequently, the sugars were determined by comparison with certain sugar samples by injecting relative response factors (Huang et al. 2017b).
The experiment shows that by using different triflates as co-catalysts, the yields of C9 lignin monomers were increased. In addition, it was observed that by using tri- and tetravalent metal triflates such as Al, Sc, La and Hf, a higher recovery of lignin monomers was achieved than by using divalent triflates such as Ni and Cu (Huang et al. 2017b). This may possibly be attributed to the fact that the cation charge increases with higher Lewis acidity. This is supported by the fact that by reference to pyridine IR, it was discovered that the Lewis acidity in ionic liquids is higher for trivalent and tetravalent metal triflates (Huang et al. 2017b). The best results with the highest yield were obtained in the experiment with Al(III) triflate. Likewise, high yielding results were obtained with Hf(IV) triflate and Yb(III) triflate. However, Yb is a rather rare metal; hence, the use of Hf and Al triflates is more profitable (Huang et al. 2017b). Considering the recovery of the methylated C5 sugars, a higher result was also obtained with Al(III) and Hf(IV) triflate. This indicates that a higher degree of hemicellulose removal was achieved. Since lignin in lignocellulose is bound to hemicellulose, it can be concluded that a higher degree of lignin monomer extraction was obtained. When the reaction time was increased from 1 to 2 h, only a slight improvement in the monomer yield was produced. Among other things, this could be due to the fact that the predominant β-O-4 ether bonds are cleaved initially, which happens quite rapidly. This type of bond is the main linkage in lignin that has to be cleaved to gain monomers. Subsequently, the remaining bonds are cleaved, which proceeds considerably slower (Huang et al. 2017b). The experiment also demonstrated that the use of the co-catalysts AlCl3 and ZnCl2 is less advantageous for the conversion of lignocellulose. Both catalysts are conventional Lewis acids (Huang et al. 2017b). Etherification and hydrogenolysis reactions produce water, which can lead to a hydrolysis reaction, reducing the efficiency of these Lewis acids (Huang et al. 2017b). Finally, it could not be evidenced that the use of hydrogen is much more beneficial for the degeneration of lignin than nitrogen, which also demonstrates that transfer hydrogenation reactors are inefficient (Huang et al. 2017b).
Lignin hydrogenolysis: Comparison of Ru/C and Pd/C catalyst
The efficiency of the Pd/C catalyst was tested by comparing its performance with that of a Ru/C catalyst under identical reaction conditions. The results are summarized in Table 24. 2 g extracted birch sawdust consisting of 19.5 wt% Klason lignin and 41/21 wt% C6/C5 sugars along with 0.2 g each of the respective catalyst were heated together with 40 mL of the solvent MeOH for 3 h at 523 K at 3 MPa hydrogen in a reactor (van den Bosch et al. 2015). Both catalysts are platinum metals. A fairly similar yield of lignin monomers of almost 50% was obtained by using both catalysts. However, a gas chromatogram showed that the product selectivity differs. The Ru/C catalyst tends to generate para-propyl phenolics. Thus, 75% of dihydroeugenol (also called 4-propylguaiacol) (1) and propylsyringol (2) were formed. The Pd/C catalyst, on the other hand, tended to form para-propanol phenolics. A majority of 91% of 4-(3-hydroxypropyl)-2-methoxyphenol (7) and 4-(3-hydroxypropyl)-2–6-dimethoxyphenol was observed. Furthermore, a remarkably similar delignification rate of around 90% could be achieved with both catalysts (van den Bosch et al. 2015). In the yield of the di- and oligomers, only a slight difference in selectivity was observed. However, somewhat larger di- and oligomers were obtained by the Pd/C catalyst. This can probably be attributed to the higher OH content of the catalyst (van den Bosch et al. 2015). The experiment has demonstrated that a high yield of different phenolic monomers can be obtained from birch wood using MeOH as solvent and a hydrogenic atmosphere. This is caused by a catalyst that induces a redox reaction. Besides phenols, additional di- and oligomers and carbohydrate pulp are also obtained under these reaction conditions. Depending on which catalyst is chosen, it is possible to control the product selectivity. In the case of lignin conversion, the Pd/C catalyst has proven to be particularly advantageous, as it allows more OH groups to be obtained (van den Bosch et al. 2015). The lignin oils obtained by the two catalysts were analyzed by 2D HSQC, 13C and 1 H NMR (van den Bosch et al. 2015). This allowed the components to be characterized and subsequently compared.
Lignin hydrogenolysis: Pd/C + CrCl3
The following experiment has the lignin conversion of the three different lignin species dealkaline lignin, sodium lignosulfonate and organosolv lignin caused by a catalytic hydrogenolysis. For this purpose, the catalyst Pd/C and in combination with the co-catalyst CrCl3 were used. For this purpose, 0.5 g each of the 5 w% Pd/C catalyst was heated with 40 mL of 1 mmol MeOH in a reactor at 4 MPa hydrogen for 5 h at 280 °C (Shu et al. 2016). The catalyst was then mixed with 1 mmol MeOH. In the co-catalyst experiments, 0.1 hg CrCl3 was also added. The results are summarized in Table 25. It can be seen that without the use of the catalyst, liquefaction could be induced in all three lignin species by the increased heat alone. For all three types, the liquefaction was around 60% (Shu et al. 2016). The product selectivity was also quite low compared to the other verses. The experiments in which the catalyst Pd/C was added showed an increased lignification. However, the selectivity with respect to aliphatic alcohols, hydrocarbons, guaiacols and phenols was only slightly increased. However, the highest yields of oligomers were obtained. By supporting the Pd/C catalyst with the co-catalyst CrCl3, a significant increase in the degree of lignification was achieved. This was around 80% for all three lignin types. The increased product yield was also remarkable, especially for the phenols. In order to determine the degree of lignin liquefaction as well as the obtained yield, the following equations were used (Shu et al. 2016):
Metal chloride cooperated with Pd/C and effect of Lewis acid
The following experiment demonstrates the effect of Lewis acidity on the degree of lignin liquefaction and on the resulting volatile products. For this purpose, 0.5 g of alkali lignin, 0.1 g of 5 wt% Pd/C catalyst, 0.5 mmol of the respective Lewis acid that was used as well as 40 mL of MeOH were heated in a 100-ml autoclave at 260 °C for 5 h. Beforehand, the autoclave was purged 3 times with hydrogen. After 5 h, the reaction was terminated by cooling the reactor to room temperature (Shu et al. 2015). The resulting product mixture was subsequently filtered. The solid fraction was rinsed 3 times with MeOH and thereafter dried at 80 °C. The remaining filtrate was collected and diluted with MeOH to a certain volume. Afterward, the quantitative and qualitative analysis was carried out using a gas chromatograph equipped with a thermal conductivity detector (TCD) and a flame ionization detector (Shu et al. 2015). Analysis of the collected gas has shown that the gaseous products were only about 1 wt% of the feed lignin, and therefore its yield was neglected here (Shu et al. 2015). For the phenolic compounds, helium was used as the carrier material, and for the volatile products, acetophenone was used as the internal standard chemical (Shu et al. 2015). The following equations were used to calculate the obtained yields and the degree of lignin liquefaction:
The findings of the experiment are shown in Table 26. The reaction conditions result in a lignification of up to 49.8% even without the use of a catalyst. This value is the lowest compared to the rest of the experiments where a catalyst was present. This also applies to the yield of phenolic monomers, which was merely 5.6%. In the second experiment, the improvement of the catalyst becomes evident as the catalyst Pd/C is added. This resulted in a higher degree of lignification, but the product yield was not particularly affected. An increase in phenol yield was achieved by adding Pd/C catalyst containing MCLx to the lignin molecules (Shu et al. 2015). This also significantly increased the degree of lignification. It should be noted that if an acidic catalyst is used, the ether bonds in the lignin, such as β-O-4 or 4-O-5, are easier to break, which further improves the liquefaction of lignin. In addition, atomic H on the surface of the Pd reduces the active energy of the ether bonds (Shu et al. 2015). Cl has a high electronegativity and is also an excellent hydrogen bond acceptor. Experiments show that by using the ionic liquid Cl, the C–O bonds in cellulose could be broken more efficiently than by using other solvents. These C–O bonds are also present in lignin molecules, which is why the use of MClx is beneficial in the conversion of lignin molecules. It can be assumed that Cl in Lewis acid acts as a hydrogen bond acceptor for lignin as well as a polarization reagent for C–O bonds in these molecules (Shu et al. 2015). All experiments carried out showed that by using a Pd/C catalyst, in which MCLx was cooperated, an increase in the degree of lignin liquefaction as well as a higher yield of phenolic products could be achieved. Furthermore, it was found in the experiment that increasing valence of the metal cation increased the lignin liquefaction. This can probably be attributed to the fact that the Lewis acid strength caused the effect by the metal cation (Shu et al. 2015). This is because the lignin hydrogenation could be promoted by the metal cations with a higher valence forming acidic centers and thus improving the acid effect of the catalyst. Among other things, therefore, the lignin depolymerization was higher when AlCl3 was used than, for example, when MgCl2 was used. It should also be noted that the higher proportion of Cl in AlCl3 also increases the depolymerization of the lignin (Shu et al. 2015). In particular, the use of LiCl resulted in a higher phenol yield compared to bivalent metal chlorides such as BaCl2 or NiCl2. Li+ molecules have the particular advantage that they have a comparatively similar radius to H+ atoms (Shu et al. 2015). Therefore, LiCl catalysis is quite similar to HCl catalysis. The main products obtained in the experiments were guaiacol and its derivatives. This is due to the fact that the lignin used consisted largely of guaiacyl units (Shu et al. 2015). However, due to the presence of CrCl3 in the Pd/C, mainly phenols were obtained as products. This is due to the fact that CrCl3 operates as a Lewis acid as well as a coordinated catalyst for C–O bonds in lignin (Shu et al. 2015). The experiment has shown that by replacing the Cl with a carbon ion or with NO3, a severe decrease in the phenolic products yielded was caused. The synergistic effect between Cl and Cr3+ was largely responsible for the increased phenolic yield. In addition to the metal chlorides, the influence of the proton acids H2SO4 and HCl was also observed. The results show that only a small amount of phenols was converted. Moreover, in the presence of HCl, a better catalytic activity was obtained than with H2SO4. This again proved that the Cl molecules have a strong effect on the depolymerization. In conclusion, the use of the Pd/C catalyst in the presence of metal chlorides increased the yield of phenolic products (Shu et al. 2015). In addition, a higher valence of the metal chloride and a stronger Lewis acidity also had a positive effect (Shu et al. 2015).
Conclusion
The various experiments in which different catalyst types were used have highlighted the fact that lignin conversion and product selectivity can be significantly influenced by the choice of the catalyst type. Different catalysts promote the formation of different products. Furthermore, it was demonstrated that factors such as temperature, solvent, Lewis acid, lignin type and use of co-catalysts can significantly improve the efficiency of the catalyst, though the influence of, i.e., the solvent cannot be generalized. For example, using Ni/C polar protic solvents seem to be beneficial to the overall yield but water as solvent seems to be problematic. If RANEY nickel is used, unpolar solvents seem to have a very positive effect on the depolymerization, resulting in overall yields up to 100%. Generally, the reaction type has to be considered. For example, zeolitic catalysts have proven to be suitable for the depolymerization of lignin by pyrolysis, whereas metal catalysts such as nickel-based catalysts are well-established for hydrogenolysis processes. Nickel catalysts are also quite efficient in the conversion of lignin to phenols. They also have the advantage of having mild reaction conditions. Another benefit of these catalysts is that they are fairly inexpensive. Pd-based catalysts have also been successfully used in the depolymerization of lignin. However, palladium is a rare metal, which is also rather expensive. Nickel, on the other hand, is an earth abundant metal. Zeolite catalysts are also less expensive than Pd catalysts and can also be used efficiently in pyrolysis processes. In addition, zeolites can be easily optimized for the process by desilication. Nonetheless, the economic aspects cannot be reduced to the costs of the corresponding catalysts. In addition, availability plays an important role. For example, established zeolites are indeed low priced, but if they have to be further processed or functionalized, they can become dull to a possible industrial user. But also the resulting product selectivity is of great importance. If many different substances have to be separated from each other, the process can become unprofitable. In our opinion, the most interesting techniques are those, where monoaromatic products can be isolated in a good yield/selectivity relation, which than can be used as basic chemicals in chemical industry replacing comparable compounds today still produced from fossil sources.
References
Adhikari S, Srinivasan V, Fasina O (2014) Catalytic pyrolysis of raw and thermally treated lignin using different acidic zeolites. Energy Fuels 28(7):4532–4538. https://doi.org/10.1021/ef500902x
Calvo-Flores FG, Dobado JA (2010) Lignin as renewable raw material. Chemsuschem 3(11):1227–1235. https://doi.org/10.1002/cssc.201000157
Font R, Esperanza M, Garcı́a AN (2003) Toxic by-products from the combustion of Kraft lignin. Chemosphere 52(6):1047–1058. https://doi.org/10.1016/S0045-6535(03)00294-7
Forchheim D, Hornung U, Kempe P, Kruse A, Steinbach D (2012) Influence of RANEY Nickel on the Formation of Intermediates in the Degradation of Lignin. Int J Chem Eng 2012:1–8. https://doi.org/10.1155/2012/589749
Geib SM, Filley TR, Hatcher PG, Hoover K, Carlson JE, Jimenez-Gasco D, Mar M et al (2008) Lignin degradation in wood-feeding insects. Proc Natl Acad Sci United States Am 105(35):12932–12937. https://doi.org/10.1073/pnas.0805257105
Goodell B (2003) Wood deterioration and preservation. Advances in our changing world. American Chemical Society; Symposium Wood Deterioration Mechanisms and Its Impact on Biotechnology and Wood Preservation; National meeting of the American Chemical Society. Washington, DC: American Chemical Society (ACS Symposium Series, 845).
Guigo N, Mija A, Vincent L, Sbirrazzuoli N (2010) Eco-friendly composite resins based on renewable biomass resources: Polyfurfuryl alcohol/lignin thermosets. Eur Polym J 46(5):1016–1023. https://doi.org/10.1016/j.eurpolymj.2010.02.010
Hoff TC, Gardner DW, Thilakaratne R, Proano-Aviles J, Brown RC, Tessonnier J-P (2017) Elucidating the effect of desilication on aluminum-rich ZSM-5 zeolite and its consequences on biomass catalytic fast pyrolysis. Appl Catal A 529:68–78. https://doi.org/10.1016/j.apcata.2016.10.009
Hosoya T, Kawamoto H, Saka S (2007) Pyrolysis behaviors of wood and its constituent polymers at gasification temperature. J Anal Appl Pyrol 78(2):328–336. https://doi.org/10.1016/j.jaap.2006.08.008
Huang S, Mahmood N, Zhang Y, Tymchyshyn M, Yuan Z, Xu C (2017a) Reductive de-polymerization of kraft lignin with formic acid at low temperatures using inexpensive supported Ni-based catalysts. Fuel 209:579–586. https://doi.org/10.1016/j.fuel.2017.08.031
Huang X, Morales Gonzalez OM, Zhu J, Korányi TI, Boot MD, Hensen EJM (2017b) Reductive fractionation of woody biomass into lignin monomers and cellulose by tandem metal triflate and Pd/C catalysis. Green Chem 19(1):175–187. https://doi.org/10.1039/C6GC02962K
Kasakov S, Shi H, Camaioni DM, Zhao C, Baráth E, Jentys A, Lercher JA (2015) Reductive deconstruction of organosolv lignin catalyzed by zeolite supported nickel nanoparticles. Green Chem 17(11):5079–5090. https://doi.org/10.1039/C5GC02160J
Kawamoto H (2017) Lignin pyrolysis reactions. J Wood Sci 63(2):117–132. https://doi.org/10.1007/s10086-016-1606-z
Klein I, Saha B, Abu-Omar MM (2015) Lignin depolymerization over Ni/C catalyst in methanol, a continuation: effect of substrate and catalyst loading. Catal Sci Technol 5(6):3242–3245. https://doi.org/10.1039/C5CY00490J
Kleinert M, Barth T (2008) Phenols from Lignin. Chem Eng Technol 31(5):736–745. https://doi.org/10.1002/ceat.200800073
Li J, Li X, Zhou G, Wang W, Wang C, Komarneni S, Wang Y (2014) Catalytic fast pyrolysis of biomass with mesoporous ZSM-5 zeolites prepared by desilication with NaOH solutions. Appl Catal A 470:115–122. https://doi.org/10.1016/j.apcata.2013.10.040
Luo H, Klein IM, Jiang Y, Zhu H, Liu B, Kenttämaa HI, Abu-Omar MM (2016) Total utilization of miscanthus biomass, lignin and carbohydrates, using earth abundant nickel catalyst. ACS Sustain Chem Eng 4(4):2316–2322. https://doi.org/10.1021/acssuschemeng.5b01776
Mihalcik DJ, Mullen CA, Boateng AA (2011) Screening acidic zeolites for catalytic fast pyrolysis of biomass and its components. J Anal Appl Pyrol 92(1):224–232. https://doi.org/10.1016/j.jaap.2011.06.001
Ouyang X, Huang X, Boot MD, Hensen EJM (2020) Efficient conversion of pine wood lignin to phenol. Chemsuschem 13(7):1705–1709. https://doi.org/10.1002/cssc.202000485
Pandey MP, Kim CS (2011) Lignin depolymerization and conversion: a review of thermochemical methods. Chem Eng Technol 34(1):29–41. https://doi.org/10.1002/ceat.201000270
Pepper JM, Lee YW (1970) Lignin and related compounds. II Studies using ruthenium and Raney nickel as catalysts for lignin hydrogenolysis. Can J Chem 48(3):477–479
Saiz-Jimenez C, de Leeuw JW (1986) Lignin pyrolysis products: Their structures and their significance as biomarkers. Org Geochem 10(4–6):869–876. https://doi.org/10.1016/S0146-6380(86)80024-9
Shu R, Long J, Yuan Z, Zhang Qi, Wang T, Wang C, Ma L (2015) Efficient and product-controlled depolymerization of lignin oriented by metal chloride cooperated with Pd/C. Biores Technol 179:84–90. https://doi.org/10.1016/j.biortech.2014.12.021
Shu R, Long J, Xu Y, Ma L, Zhang Qi, Wang T et al (2016) Investigation on the structural effect of lignin during the hydrogenolysis process. Biores Technol 200:14–22. https://doi.org/10.1016/j.biortech.2015.09.112
Singh SK, Ekhe JD (2015) Cu–Mo doped zeolite ZSM-5 catalyzed conversion of lignin to alkyl phenols with high selectivity. Catal Sci Technol 5(4):2117–2124. https://doi.org/10.1039/C4CY01700E
Song Qi, Wang F, Cai J, Wang Y, Zhang J, Yu W, Xu J (2013) Lignin depolymerization (LDP) in alcohol over nickel-based catalysts via a fragmentation–hydrogenolysis process. Energy Environ Sci 6(3):994. https://doi.org/10.1039/c2ee23741e
van den Bosch S, Schutyser W, Koelewijn S-F, Renders T, Courtin CM, Sels BF (2015) Tuning the lignin oil OH-content with Ru and Pd catalysts during lignin hydrogenolysis on birch wood. Chem Commun (cambridge, England) 51(67):13158–13161. https://doi.org/10.1039/c5cc04025f
Vankoningsveld H, Jansen J, Vanbekkum H (1990) The monoclinic framework structure of zeolite H-ZSM-5. Comparison with the orthorhombic framework of as-synthesized ZSM-5. Zeolites 10(4):235–242. https://doi.org/10.1016/0144-2449(94)90134-1
Wang X, Rinaldi R (2012) Solvent effects on the hydrogenolysis of diphenyl ether with Raney nickel and their implications for the conversion of lignin. Chemsuschem 5(8):1455–1466. https://doi.org/10.1002/cssc.201200040
Wang H, Tucker M, Ji Y (2013) Recent development in chemical depolymerization of lignin: a review. J Appl Chem 2013:1–9. https://doi.org/10.1155/2013/838645
Windeisen E, Wegener G (2008) Behaviour of lignin during thermal treatments of wood. Indus Crops Prod 27(2):157–162. https://doi.org/10.1016/j.indcrop.2007.07.015
Yang H, Yan R, Chen H, Lee DH, Zheng C (2007) Characteristics of hemicellulose, cellulose and lignin pyrolysis. Fuel 86(12–13):1781–1788. https://doi.org/10.1016/j.fuel.2006.12.013
Zhao C, Kou Y, Lemonidou AA, Li X, Lercher JA (2010) Hydrodeoxygenation of bio-derived phenols to hydrocarbons using RANEY® Ni and Nafion/SiO2 catalysts. Chem Commun. https://doi.org/10.1039/B916822B
Acknowledgements
We like to thank the ReAching program of the faculty MLS, Furtwangen University, for support.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Gücyeter, S., Erpelding, R. & Schmidt, M.S. Review: chemical approaches toward catalytic lignin degradation. Chem. Pap. 76, 1899–1922 (2022). https://doi.org/10.1007/s11696-021-01996-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11696-021-01996-y